Staff Writer, TMN Jul 03, 2018 6 years, 9 months, 1 week, 4 days, 23 hours, 11 minutes ago
Some researchers believe they will soon be able to slow or even stop the body's clock—at least for a little while
The majority of older people live out their final years with at least one or two chronic ailments, such as arthritis, diabetes, heart disease or stroke. The longer their body clock ticks, the more disabling conditions they face.
Doctors and drug companies traditionally treat each of these aging-related diseases as it arises. But a small group of scientists have begun championing a bold new approach. They think it is possible to stop or even rewind the body's internal chronometer so that all these diseases will arrive later or not at all.
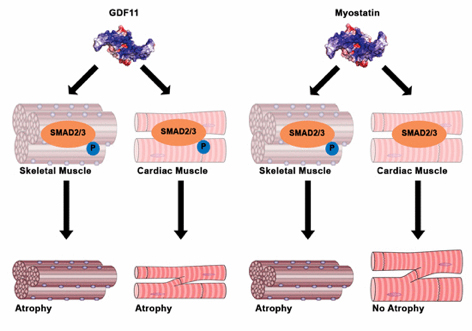
Growth and differentiation factor (GDF) 11 is a recently suggested anti‐ aging therapeutic
Studies of centenarians suggest the feat is achievable. Most of these individuals live that long because they have somehow avoided most of the diseases that burden other folks in their 70s and 80s, says Nir Barzilai, director of the Institute for Aging Research at the Albert Einstein College of Medicine. Nor does a centenarian's unusual longevity result in an end-of-life decline that lasts longer than anyone else's. In fact, Barzilai notes, research on hundreds of “super agers” suggests exactly the opposite. For them, illness typically starts later and arrives closer to the end. “They live, live, live and then die one day,” he says.
Researchers have already developed various techniques to increase the life span of yeast, worms, flies, rats and perhaps monkeys. Adapting these measures to people seems like the next logical step. “There's an emerging consensus that it's time to take what we've learned from aging [research] and begin to translate that into helping humans,” says Brian Kennedy, CEO and president of the Buck Institute for Research on Aging, an independent research group in Novato, Calif.
Delaying the aging process by even a few years could offer enormous social benefits as populations around the globe grow increasingly older. The U.S. Census Bureau estimates that one in five Americans will be older than 65 by 2030—up from one in seven in 2014. In 2013 an estimated 44 million people around the world suffered from dementia. That number is expected to jump to nearly 76 million in 2030 and 135 million in 2050—with not nearly enough younger people in a position to be able to take care of them.
Among the handful of approaches that researchers are studying, three stand out. Still unclear: whether the potential benefits outweigh the risks of the treatments.
EVIDENCE
Of course, to conclusively determine whether a treatment works, investigators need a definition of aging and a way to measure the process. They have neither. If a kidney cell divided yesterday, is it one day old or as old as the person in whom it resides? Still, research over the past decade has offered several hints that the damaging aspects of aging—however you define it—can be slowed.
In a 2005 study, Thomas Rando, director of the Paul F. Glenn Center for the Biology of Aging at Stanford University, showed that an elderly mouse whose bloodstream was surgically linked to a young mouse recovered its youthful wound-healing powers. Somehow the older rodent's stem cells, w
hich are responsible for replacing damaged cells, became more effective at giving rise to new tissue. Harvard University biologist Amy Wagers has since found a protein, dubbed GDF11, in the blood that may have contributed to the faster healing. Her experiments, published in
Science in 2014, found more of the protein in younger mice than in older ones; when injected in older mice, GDF11 appeared to restore muscles to their youthful structure and strength. A new study, in
Cell Metabolism, calls that finding into question, however, suggesting that GDF11 increases with age (and may even inhibit muscle restoration) and that some other factor must make the cells act younger.
A second approach consists of examining about 20 currently existing medications and nutritional supplements at a level of detail that has never before been possible to see whether they might actually affect the aging process. For example, researchers at Cardiff University in Wales and their colleagues reported in 2014 that patients with
type 2 diabetes who took the drug metformin lived, on average, 15 percent longer than a group of healthy people who did not suffer from the metabolic disorder but were similar in nearly all other respects. Scientists speculate that metformin interferes with a normal aging process, called glycation, in which glucose combines with proteins and other important molecules, gumming up their normal workings. The metformin finding is particularly striking because people who have diabetes, even if it is well controlled, typically have somewhat shorter life spans than their healthy counterparts.
Meanwhile, in a study of 218 adults published late last year in
Science Translational Medicine researchers at pharmaceutical company Novartis showed that a compound called everolimus, which is chemically similar to rapamycin (a drug used to prevent kidney rejection in transplants), improved the effectiveness of the flu shot in people older than 65.
As individuals age, their immune systems do not mount as strong an antibody response to the inactivated virus in the vaccine as they once did; thus, older people are more likely to get sick if they later encounter a real flu virus. Tests showed that study patients given everolimus had a higher concentration of germ-fighting antibodies in their blood than their untreated counterparts. Investigators interpreted this finding as a sign that the drug had rejuvenated the subjects' immune systems.
As with any drug, side effects were an issue. Members of the treated group were more likely to develop ulcers in their mouth, which may limit the widespread usefulness of the medication for treating aging. Cost may be another factor; everolimus, which was approved by the U.S. Food and Drug Administration for its cancer-fighting properties, costs more than US$7,000 a month at doses appropriate for cancer. Not yet known: how much everolimus would cost and how long it would be needed, if used as an antiaging drug.
Nevertheless, the results support the idea that aging can be slowed. Indeed, everolimus and other rapamycinlike drugs have been shown to dramatically extend the life span of mice, preventing diseases such as cancer and reversing age-related changes to the blood, liver, metabolism and immune system.
A third, completely different approach involves diet. Restricting the consumption of calories was long ago shown to help mice to live longer. Whether limiting food intake (without causing malnutrition) might benefit humans as well is not so clear. For one thing, very few people can or want to maintain such low-calorie diets for the decades needed to prove definitively that this approach works. But it may turn out that such drastic steps are unnecessary. Valter Longo, director of the Longevity Institute at the University of Southern California, has shown that he can extend the life span of mice merely by limiting their food on alternate days or by cutting down on the amount of protein they consume. Such intermittent fasting may turn out to be more palatable for people, although its benefits remain unproved.
CAVEATS
Living longer may come with trade-offs. Making old cells young again will mean they will start dividing again. Controlled cell division equals youthfulness; uncontrolled cell division equals cancer. But at the moment, scientists are not sure if they can do one without the other.
Figuring out the right timing for treatment is also complicated. If the goal is to prevent multiple diseases of aging, do you start your antiaging therapies when the first disease hits? The second? “Once you're broken, it's really hard to put you back together. It's going to be easier to keep people healthy,” Kennedy says. So it probably makes more sense to start treatment years earlier, during a healthy middle age. But the research needed to prove that supposition would take decades.
If various diseases can be pushed off, the next obvious question is by how long. James Kirkland, who directs the Mayo Clinic's Robert and Arlene Kogod Center on Aging in Rochester, Minn., says it will take at least another 20 years of study to answer that question. Scientists have successfully extended the life span of worms eightfold and added a year of life to three-year-old lab mice. Would these advances translate into an 80-year-old person living five or six centuries or even an extra 30 years? Or would they get just one more year? Life extension in people is likely to be more modest than in yeast, worms, flies or mice, Rando says. Previous research has suggested that lower-order creatures benefit the most from longevity efforts—with yeast, for instance, deriving a greater benefit in caloric-restriction experiments than mammals. “The closer you get to humans, the smaller the effect” on life span, he says. And what magnitude of benefit would someone need to justify taking—and paying for—such a treatment? “Do you take a drug your whole life hoping to live 4 percent longer or 7 percent longer?” Rando asks.
What, if anything, do antiaging investigators themselves do to try to slow their own aging? The half a dozen scientists interviewed for this article all said that they make concerted efforts to extend their own life span. One was grateful for a diagnosis of prediabetes, which meant a legitimate prescription for metformin. The research is getting so solid, Kennedy says, that he is having a tougher time convincing himself not to take some drugs than to take them.
All the experts say they try to live healthy lives, aside from enduring high-pressured jobs. They try to get close to eight hours of sleep, eat moderate amounts of nutritious foods and get lots of exercise. None of them smokes. Most Americans, unfortunately, do not follow such healthy habits. The greatest irony would be to discover that a pill is not, in the end, any more effective than the healthy habits we already ignore.