89Zr-immuno-PET, New Non-Invasive Way To Predict Effectiveness Of Cancer Therapy
Source: Thailand Medical News Dec 14, 2019 5 years, 4 months, 1 week, 6 days, 12 hours, 54 minutes ago
Medical and
imaging researchers have taken a critical step toward developing a
non-invasive nuclear medicine technique that can predict the effectiveness of therapy for cancerous tumors, allowing for personalized, precision treatment. The study is featured in the December issue of The
Journal of Nuclear Medicine.
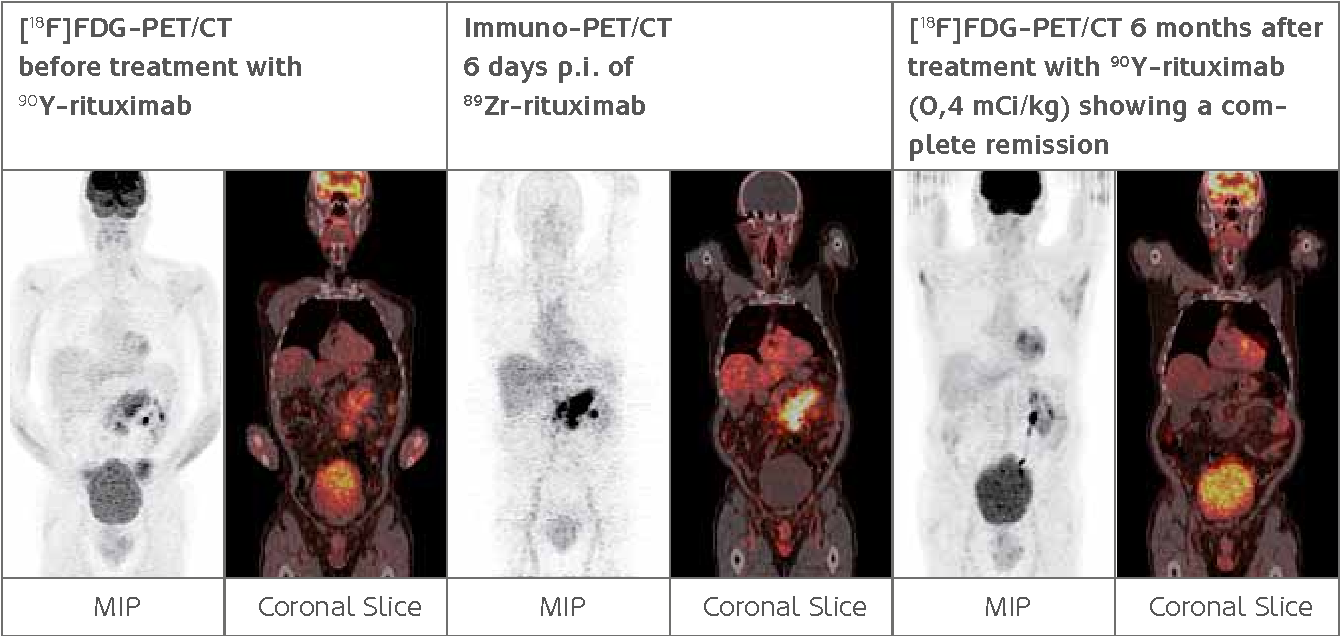
The
89Zr-immuno-PET is a noninvasive, whole-body imaging technique with the potential to predict the effectiveness of therapeutic antibodies (or their conjugates) in treating tumors. This is a significant advance: currently, the only ways to measure this are through tissue sampling, which is invasive and noncomprehensive, or by measuring concentrations of monoclonal antibodies in blood samples.
Dr Yvonne Jauw, MD, at the Cancer Center Amsterdam, Amsterdam UMC in The Netherlands told
Thailand Medical News, "This study provides proof of concept that
PET imaging with
89Zr-labeled antibodies can be used to assess physiological components of antibody biodistribution. This research enables us to apply molecular imaging as a
noninvasive clinical tool to measure antibody concentrations in normal tissues."
For this retrospective analysis of clinical
89Zr-immuno-PET studies, data from 128 PET scans were collected from Amsterdam UMC; CHU Lille in Lille, France; and Memorial Sloan Kettering
Cancer Center in New York, New York. The scans were of 36 patients and were done one to seven days after injection with the appropriate 89Zr-labeled antibodies for imaging their tumors. Nonspecific uptake was defined as uptake measured in tissues without known target expression (normal tissue).
The study findings show that imaging with
89Zr-immuno-PET can be used to optimize detection of tumors throughout a patient's body. Nonspecific uptake of monoclonal antibodies in tissues without target expression can be quantified using
89Zr-immuno-PET at multiple time points. These results form a crucial base for measurement of target engagement by therapeutic antibodies in a living person. For future studies, a pilot phase, including at least three scans at one or more days after injection, is needed to assess nonspecific uptake as a function of time and to optimize study design for detection of target engagement and effectiveness against tumors.
The research has important implications for patients, as JDr auw points out: "Knowledge of antibody distribution to normal tissues and tumors can be used to increase our understanding of which drugs will be effective and which drugs are likely to cause toxicity."
In the near future, this clinical tool could also be used in the selection of monoclonal antibodies during drug development, as well as the selection of patients who could benefit from a specific treatment.
Reference
rong> : Yvonne W.S. Jauw et al, 89Zr-Immuno-PET: Toward a Noninvasive Clinical Tool to Measure Target Engagement of Therapeutic Antibodies In Vivo, Journal of Nuclear Medicine (2019). DOI: 10.2967/jnumed.118.224568 