A new hope in cancer treatment, harnessing lactate oxidase to disrupt cancer cell survival mechanisms
Nikhil Prasad Fact checked by:Thailand Medical News Team Aug 13, 2024 8 months, 1 week, 6 days, 7 hours, 33 minutes ago
Cancer News: Recent research from Washington State University, along with contributions from various institutions including the Everett Clinic and Eastern Washington University, has shed light on a novel approach to cancer treatment. This study focuses on using an enzyme, lactate oxidase (LOX), to disrupt critical survival mechanisms in breast cancer cells. The findings of this study could pave the way for new therapeutic strategies that target cancer at a molecular level. This
Cancer News report delves into the details of how LOX interacts with cancer cells and its potential implications for future treatments.
 A new hope in cancer treatment, harnessing lactate oxidase to disrupt cancer cell survival mechanisms
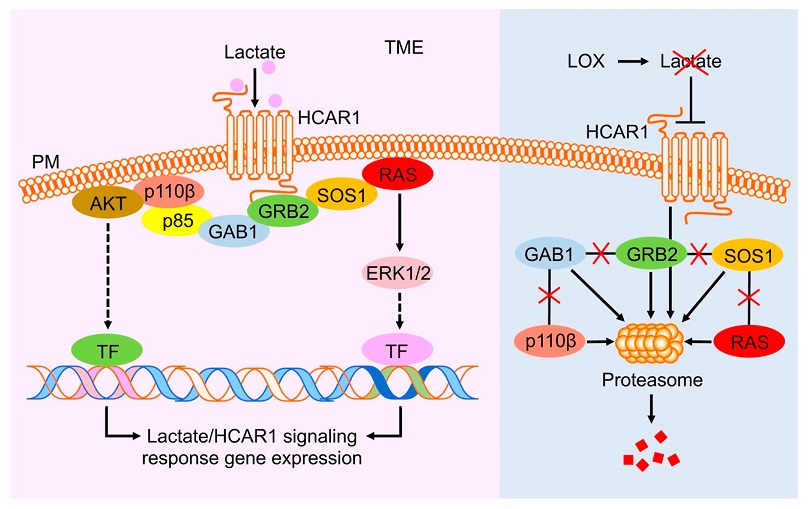
Model of lactate and LOX regulation of the HCAR1-RAS/PI3K signaling cascades. TME lactate activates HCAR1, which induces the assembly of a protein complex that includes the receptor itself, GRB2, SOS1, RAS, GAB1, p85, and p110β. Subsequently, the RAS and PI3K pathways are activated and transduce the lactate-triggered signals, via phosphorylated ERK1/2 and AKT, respectively, to the downstream transcription factors that further mediate the transcription of the genes involved in promoting the signaling and cancer cell survival and growth (left panel). Introducing LOX to the TME depletes lactate and renders HCAR1 inactive for triggering the assembly of the RAS and PI3K signaling complex. The disassembled HCAR1-associated RAS and PI3K signaling proteins are targeted for degradation by proteasomes (right panel). TME, tumor microenvironment; PM, plasma membrane; TF, transcription factor; the criss cross lines in the right panel represent removal or loss of interaction or signaling.
The Role of Lactate in Cancer Survival
A new hope in cancer treatment, harnessing lactate oxidase to disrupt cancer cell survival mechanisms
Model of lactate and LOX regulation of the HCAR1-RAS/PI3K signaling cascades. TME lactate activates HCAR1, which induces the assembly of a protein complex that includes the receptor itself, GRB2, SOS1, RAS, GAB1, p85, and p110β. Subsequently, the RAS and PI3K pathways are activated and transduce the lactate-triggered signals, via phosphorylated ERK1/2 and AKT, respectively, to the downstream transcription factors that further mediate the transcription of the genes involved in promoting the signaling and cancer cell survival and growth (left panel). Introducing LOX to the TME depletes lactate and renders HCAR1 inactive for triggering the assembly of the RAS and PI3K signaling complex. The disassembled HCAR1-associated RAS and PI3K signaling proteins are targeted for degradation by proteasomes (right panel). TME, tumor microenvironment; PM, plasma membrane; TF, transcription factor; the criss cross lines in the right panel represent removal or loss of interaction or signaling.
The Role of Lactate in Cancer Survival
Cancer cells are known for their high metabolic activity, which results in the production of large amounts of lactate. This lactate is not merely a waste product; it plays a crucial role in supporting cancer cell survival and proliferation. Lactate acts as a signaling molecule, activating the hydroxycarboxylic acid receptor 1 (HCAR1) on cancer cells. This activation leads to the assembly of a protein complex that includes key players in the RAS and PI3K signaling pathways, both of which are essential for cancer cell survival and growth.
How LOX Disrupts Cancer Cell Signaling
The study conducted by researchers at Washington State University and other institutions demonstrated that lactate oxidase could disrupt this lactate-mediated signaling in breast cancer cells. LOX, an enzyme not found in mammalian cells, catalyzes the conversion of lactate to pyruvate and hydrogen peroxide (H2O2). This conversion not only depletes lactate levels in the tumor microenvironment (TME) but also generates H2O2, which induces oxidative stress and cancer cell death.
The research highlighted a novel signaling mechanism where lactate, through HCAR1 activation, promotes the assembly of a complex of proteins that drive the RAS and PI3K oncogenic pathways. By breaking down lactate, LOX effectively disrupts this
signaling nexus, leading to the degradation of key proteins involved in these pathways.
Key Findings of the Study
The study's findings are significant in several ways:
-Lactate Depletion: LOX was shown to significantly reduce lactate levels in the TME, which is crucial for cancer cell survival. By removing this essential metabolite, LOX starves the cancer cells of a key energy source.
-Disruption of Oncogenic Signaling: The research demonstrated that LOX treatment leads to the degradation of proteins involved in the RAS and PI3K pathways. These pathways are critical for cancer cell survival, and their disruption can halt tumor growth.
-Proteasomal Degradation: LOX not only reduces protein levels by preventing their synthesis but also promotes their breakdown through proteasomal degradation. This dual action further enhances its effectiveness in inhibiting cancer cell survival.
-Selective Targeting: The study found that LOX specifically targets fast-growing cancer cells while having minimal effects on normal cells. This selectivity is crucial for developing therapies that minimize damage to healthy tissues.
The Mechanism in Detail
The research revealed that lactate stimulation increases the expression of HCAR1 and its associated proteins, leading to the activation of the RAS and PI3K signaling pathways. These pathways are known to promote cancer cell survival, proliferation, and metastasis. However, when LOX is introduced, it breaks down lactate, which in turn disrupts the assembly of the HCAR1-associated protein complex. Without this complex, the RAS and PI3K pathways cannot function properly, leading to reduced cancer cell survival.
Moreover, LOX was found to induce the degradation of these proteins via the proteasome, a cellular structure responsible for breaking down unneeded or damaged proteins. This degradation further reduces the levels of proteins necessary for the RAS and PI3K pathways, effectively silencing these oncogenic signals.
Implications for Future Cancer Therapies
The implications of this study are profound. By targeting lactate, a molecule that plays multiple roles in cancer cell survival, LOX offers a multi-faceted approach to cancer treatment. This enzyme not only depletes an essential energy source for cancer cells but also disrupts critical signaling pathways that these cells rely on to survive and proliferate.
The study suggests that targeting HCAR1 and its associated signaling pathways upstream of RAS and PI3K could be a viable strategy for developing new cancer therapies. Since LOX selectively affects cancer cells while sparing normal cells, therapies based on this enzyme could offer a more targeted approach to cancer treatment, reducing the side effects commonly associated with traditional chemotherapy and radiation.
Conclusion: A Promising Future for LOX-Based Therapies
In conclusion, the research conducted by the team from Washington State University and collaborating institutions has unveiled a promising new avenue for cancer treatment. By leveraging the unique properties of lactate oxidase, this approach targets cancer cells at multiple levels, disrupting their energy supply and critical survival mechanisms. As this study highlights, LOX-based therapies have the potential to revolutionize cancer treatment by offering a more targeted and effective solution with fewer side effects.
The study findings were published in the peer-reviewed journal: Cancers.
https://www.mdpi.com/2072-6694/16/16/2817
For the latest
Cancer News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/study-finds-that-millennials-and-gen-x-are-at-higher-risk-for-17-types-of-cancer-unlike-generations-before-them
https://www.thailandmedical.news/news/californian-study-finds-that-cannabis-significantly-increases-risk-of-head-and-neck-cancer
