American Medical Experts Warn Of Enterovirus-D68 Resurgence That Can Cause Severe Respiratory And Neurological Disease In Children
Nikhil Prasad Fact checked by:Thailand Medical News Team Feb 16, 2024 1 year, 2 months, 1 week, 3 days, 15 hours, 4 minutes ago
Medical News: In recent years, American scientists have sounded the alarm on the resurgence of Enterovirus-D68 (EV-D68), a non-polio enterovirus causing severe respiratory and neurological diseases in children. Institutions such as the Uniformed Services University in Bethesda, USA, and The Children’s Hospital Colorado, along with the University of Colorado School of Medicine, have been at the forefront of studying this pathogen.
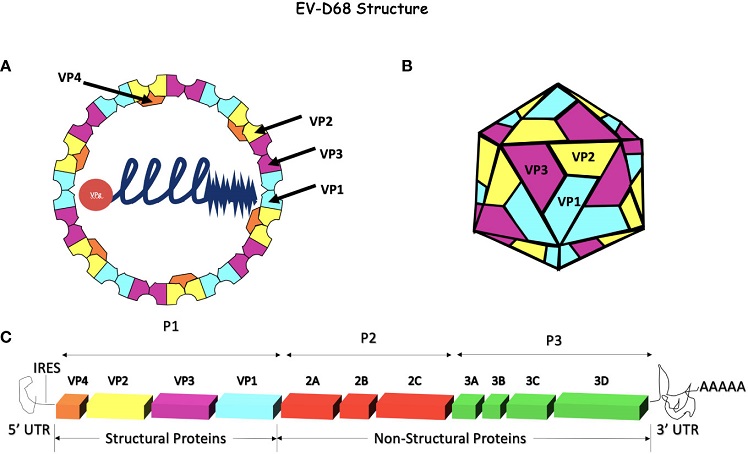 Enterovirus-D68 Genome and capsid. (A, B) EV-D68 has an icosahedral, non-enveloped capsid consisting of 4 structural proteins, VP1, VP2, and VP3 on the external side of the capsid and VP4 on the internal side. This capsid surrounds the +ssRNA naked genome attached to VPg. (C) The EV-D68 genome encodes for 4 structural proteins (VP1 - 4) and 7 non-structural proteins (2A - C and 3A - D). The internal ribosome entry site (IRES) is at the 5’ end and a poly-A tail terminates the 3’ end. The genome is ~7.2 kb in size and is composed of a single open reading frame (ORF). Initially, the polyprotein is processed into 3 precursor proteins, P1-P3. P1 is later proteolytically cleaved into the 4 structural proteins (VP1 - 4) while P2 and P3 are processed into replicase, VPg, proteases (2A and 3C), a polymerase (3D), and other non-structural proteins.
Enterovirus-D68 Genome and capsid. (A, B) EV-D68 has an icosahedral, non-enveloped capsid consisting of 4 structural proteins, VP1, VP2, and VP3 on the external side of the capsid and VP4 on the internal side. This capsid surrounds the +ssRNA naked genome attached to VPg. (C) The EV-D68 genome encodes for 4 structural proteins (VP1 - 4) and 7 non-structural proteins (2A - C and 3A - D). The internal ribosome entry site (IRES) is at the 5’ end and a poly-A tail terminates the 3’ end. The genome is ~7.2 kb in size and is composed of a single open reading frame (ORF). Initially, the polyprotein is processed into 3 precursor proteins, P1-P3. P1 is later proteolytically cleaved into the 4 structural proteins (VP1 - 4) while P2 and P3 are processed into replicase, VPg, proteases (2A and 3C), a polymerase (3D), and other non-structural proteins.
EV-D68, initially isolated in 1962 from children with pneumonia, has witnessed sporadic cases and small outbreaks over the years. However, the past decade has seen a global reemergence and rapid spread of the virus, leading to major outbreaks every other year, with more than 90% of cases occurring in children under the age of 16. The pattern was disrupted during the COVID-19 pandemic, but with the relaxation of social distancing protocols, a resurgence occurred in 2022 as reported in past
Medical News coverages.
https://www.thailandmedical.news/news/bombshell-revelation-health-officials-in-wales-hid-deadly-pediatric-enterovirus-outbreak-for-nearly-a-year
https://www.thailandmedical.news/news/outbreak-news-taiwan-facing-a-deluge-multiple-enterovirus-strains-at-epidemic-proportions
Virology
EV-D68 belongs to the Enterovirus genus of the Picornaviridae family, which also includes polioviruses, Coxsackie A and B viruses, and echoviruses. The virus has a positive-sense, single-stranded RNA genome of approximately 7.2 kb, encoding both structural and non-structural proteins. The structural proteins (VP 1-4) form the non-enveloped icosahedral capsid, while the non-structural proteins play vital roles in replication.
The virus's replication begins with binding to host cell rece
ptors, and though the exact receptor for EV-D68 is still under investigation, studies suggest involvement of sialic acid, ICAM-5, or sulfated glycosaminoglycans. The virus undergoes a structural reorganization step within endosomes, leading to the release of the viral genome into the host cell cytoplasm. Translation, proteolytic processing, and replication then follow, resulting in the assembly of new viral particles.
Epidemiology
Children, especially those under the age of 16, are most susceptible to severe EV-D68 infection. The virus predominantly circulates in temperate climates during the summer-fall season, correlating with spikes in Acute Flaccid Myelitis (AFM) cases. Notably, the virus exhibits a biennial pattern, with major outbreaks reported in 2014, 2016, and 2018.
The epidemiological landscape changed temporarily during the COVID-19 pandemic, with a decrease in EV-D68 cases. However, since the relaxation of pandemic-related measures, a resurgence has been observed in 2022, raising concerns among scientists.
Virus Structure, Replication, and Challenges
EV-D68 replication is temperature and pH-sensitive, replicating efficiently at 33°C. The virus exhibits a unique phenotypic similarity to rhinoviruses, causing primarily respiratory diseases. Recent strains show an increased ability to cause systemic infection and neuroinvasion.
Challenges in understanding EV-D68 include identifying the entry receptor, variations in strains, and the factors contributing to increased severity in specific age groups. The knowledge gaps in the field hinder the development of effective therapeutic strategies and vaccines.
Pathogenesis
EV-D68 primarily infects the respiratory tract, causing symptoms such as runny nose, coughing, and wheezing. In severe cases, especially in children below five years, it can lead to respiratory distress and acute flaccid myelitis (AFM), characterized by sudden muscle weakness, compromised reflexes, and, in extreme cases, respiratory failure.
Pathological changes in the respiratory tract are challenging to study due to the transient nature of infection. Experimental infection in animal models, such as ferrets, has provided insights into the inflammatory responses and histological evidence of interstitial pneumonia.
Central Nervous System Involvement
EV-D68 has been associated with various neurological complications, including AFM, cranial nerve dysfunction, encephalitis, and meningoencephalitis. AFM, in particular, progresses rapidly, leading to muscle weakness, respiratory distress, and in severe cases, ventilator support.
The exact mechanisms of how EV-D68 enters the central nervous system (CNS) are still unclear. Studies suggest retrograde axonal transport from peripheral nerves in the respiratory tract to the spinal cord. Additionally, viral replication in motor neurons and the presence of EV-D68 in cerebrospinal fluid (CSF) hint at potential blood-brain barrier breach.
Extra-Respiratory Spread
While EV-D68 primarily targets the respiratory tract, the virus has been detected in blood, sera, stool samples, and CSF. The mechanism of extra-respiratory spread remains unknown. Animal studies and in vitro experiments indicate potential dissemination to various tissues, including the liver, kidneys, muscles, and spinal cord.
Treatment and Vaccines
As of now, there are no approved antiviral therapies, monoclonal antibodies, or vaccines specific to EV-D68. The lack of targeted treatment options underscores the urgency to unravel the virus's complexities and develop effective countermeasures.
Conclusion
EV-D68 poses a significant public health challenge, particularly for children, with a resurgence in recent years demanding increased attention and research. While strides have been made in understanding the virology, pathogenesis, and epidemiology, numerous challenges persist. The need for effective surveillance, diagnostics, and a comprehensive understanding of immune responses remains paramount for developing strategies to control future outbreaks.
The complexities surrounding EV-D68 underscore the importance of continued research, international collaboration, and the development of targeted interventions. Addressing gaps in knowledge will be critical in mitigating the impact of this reemerging pathogen on global pediatric health.
The study findings were published in the peer reviewed journal: Frontiers in Virology.
https://www.frontiersin.org/articles/10.3389/fviro.2024.1328457/full
For the latest
Medical News, keep on logging to Thailand Medical News.
https://www.thailandmedical.news/news/virus-identified-for-disease-that-paralyses-children-and-warning-that-next-seasonal-attack-could-be-severe
https://www.thailandmedical.news/news/breaking-news-covid-19-research-new-study-indicates-that-covid-19-patients-could-develop-acute-myelitis
https://www.thailandmedical.news/news/warning-new-emerging-mutated-strain-of-flu-virus-causing-fatal-acute-flaccid-myelitis-afm
https://www.thailandmedical.news/news/scientists-baffled-by-cause-of-seasonal-afm-(acute-flaccid-myelitis)-that-causes-paralysis-in-individuals-but-also-fears-that-more-global-outbreaks-ar
