American Scientists Discover That Transposons Regulate Interferon Signaling During SARS-CoV-2 Infection
Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 15, 2024 1 year, 3 weeks, 2 days, 9 hours, 27 minutes ago
Medical News: A groundbreaking study by researchers from the University of Colorado Boulder, Fred Hutchinson Cancer Research Center, Johns Hopkins University, and other leading institutions has unveiled new insights into how transposable elements, often referred to as "jumping genes," influence the human immune system. Their research sheds light on the role of transposons in the regulation of interferon signaling, a key defense mechanism against viruses like SARS-CoV-2. This
Medical News report delves into the study findings as to how these genetic elements shape immune responses, opening doors to potential therapeutic advancements.
 American Scientists Discover That Transposons Regulate Interferon Signaling During SARS-CoV-2 Infection
Understanding Transposons and Interferon Signaling
American Scientists Discover That Transposons Regulate Interferon Signaling During SARS-CoV-2 Infection
Understanding Transposons and Interferon Signaling
Transposons, which make up more than 50% of the human genome, were long considered "junk DNA." However, recent discoveries have highlighted their significant role in regulating immune functions. The innate immune system - our first line of defense against pathogens - relies on tight regulation to combat infections while avoiding excessive inflammation or autoimmune reactions. Dysregulation of these pathways can lead to conditions like rheumatoid arthritis, lupus, and severe COVID-19.
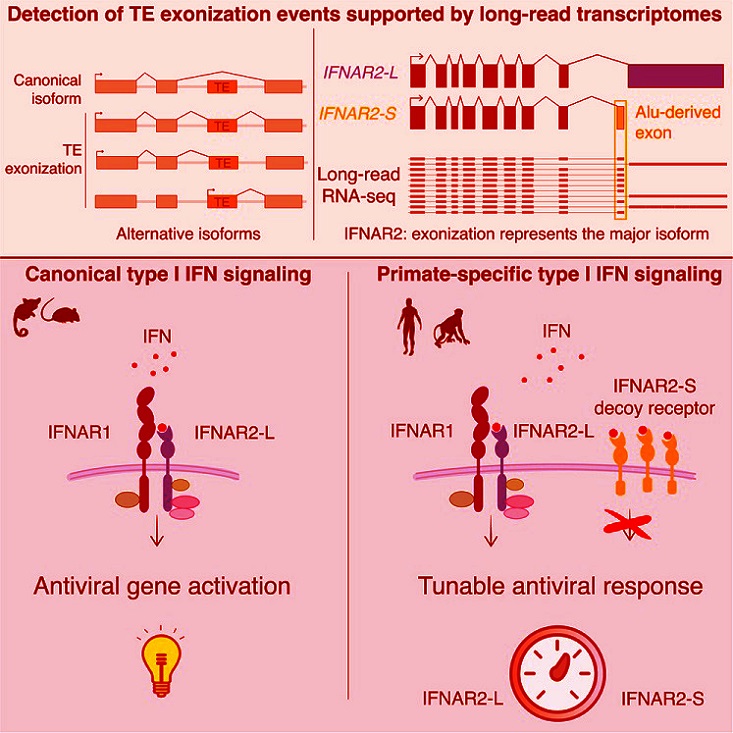
The study also explores insights from long-read transcriptome datasets that revealed how transposons contribute to the generation of alternative protein isoforms of immune genes. These include a variant of the type I interferon receptor, IFNAR2, which plays a crucial role in immune signaling. One specific discovery - a primate-specific isoform of IFNAR2 called IFNAR2-S - demonstrates how transposons have been co-opted to regulate immune responses.
The Discovery of IFNAR2-S
The study identified numerous instances of transposon-derived exons being spliced into immune genes, a process termed "exonization." The researchers focused on IFNAR2, a receptor crucial for type I interferon signaling, which is activated during viral infections such as SARS-CoV-2. IFNAR2-L, the canonical isoform, signals cells to activate antiviral defenses. However, IFNAR2-S, which lacks the intracellular signaling domain, was found to act as a decoy receptor.
By binding interferons without triggering downstream signaling, IFNAR2-S effectively dampens immune responses. This mechanism ensures that the immune system does not overreact, a vital feature for preventing inflammatory diseases. Strikingly, IFNAR2-S was expressed at higher levels than the canonical IFNAR2-L in most human tissues, underscoring its physiological importance.
Key Findings from the Study
The study’s comprehensive analysis revealed the following key findings:
-Prevalence of Transposon Exonization: Using advanced transcriptomic technologies, the researchers identified over 3,500 exonization events across 2,700 genes. Among these, immune-related genes were particularly enriched with tr
ansposon-derived exons, suggesting their evolutionary importance in immune regulation.
-Evolutionary Origins of IFNAR2-S: IFNAR2-S arose from the exonization of an Alu transposon approximately 60-70 million years ago during the evolution of simian primates. This evolutionary event added a unique dimension to the human immune system, differentiating it from other mammals.
-Functional Role of IFNAR2-S: The research demonstrated that IFNAR2-S functions as a potent inhibitor of interferon signaling. Experiments using CRISPR-edited human cell lines showed that cells lacking IFNAR2-S exhibited exaggerated responses to interferons, leading to heightened immune activation. Conversely, cells with overexpressed IFNAR2-S displayed muted responses, highlighting its regulatory role.
-Impact on SARS-CoV-2 Responses: In experiments involving SARS-CoV-2, IFNAR2-S was shown to reduce the antiviral effects of interferons. Cells lacking this isoform exhibited stronger interferon responses and significantly lower levels of viral replication. This finding implicates IFNAR2-S in modulating immune responses during COVID-19, where excessive inflammation often contributes to severe outcomes.
Clinical Implications
The discovery of IFNAR2-S has profound implications for understanding and treating immune-related diseases. Conditions like lupus, multiple sclerosis, and severe COVID-19 involve dysregulated interferon signaling. By targeting the balance between IFNAR2-S and IFNAR2-L, therapies could be developed to fine-tune immune responses.
Moreover, genetic variants in the IFNAR2 region have been linked to susceptibility to severe COVID-19. The study found that certain variants associated with lower IFNAR2-L expression and higher IFNAR2-S levels correlated with poor outcomes. This suggests that the relative expression of these isoforms could serve as a biomarker for disease severity or treatment response.
Conclusions
This research highlights the pivotal role of transposons in shaping human immunity. IFNAR2-S, derived from a primate-specific transposon, acts as a crucial regulator of interferon signaling, balancing immune activation and suppression. These findings underscore the importance of genetic diversity in immune system evolution and its implications for human health.
The study also opens avenues for future research. Understanding how IFNAR2-S modulates responses to different pathogens could lead to novel treatments for infectious diseases and autoimmune conditions. Additionally, investigating other transposon-derived isoforms may uncover further layers of immune regulation.
As the authors note, this work demonstrates how elements once considered genomic "parasites" have been repurposed to serve vital functions in human biology. By unraveling the complexities of these mechanisms, scientists can develop strategies to harness them for therapeutic benefit.
The study findings were published in the peer-reviewed journal: Cell.
https://www.cell.com/cell/fulltext/S0092-8674(24)01333-3
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/role-of-mast-cells-and-basophils-in-fighting-major-viral-infections-including-covid-19
https://www.thailandmedical.news/news/cornell-study-finds-that-glutamine-metabolism-is-key-to-coronavirus-survival
https://www.thailandmedical.news/articles/coronavirus
Follow Us on:
https://x.com/ThailandMedicaX
https://bsky.app/profile/thailandmedical.bsky.social
https://www.facebook.com/ThailandMedicalNews
https://gettr.com/user/thailandmedicalnews
https://www.tribel.com/thailandmedical/wall
