Nikhil Prasad Fact checked by:Thailand Medical News Team Jun 04, 2024 10 months, 3 weeks, 1 day, 19 hours, 45 minutes ago
COVID-19 News: The ongoing battle against COVID-19 has witnessed significant strides in understanding the virus and developing vaccines and treatments. Among the latest findings, a team from the 'Federico II' University of Naples and CEINGE Biotecnologie Avanzate in Naples, Italy, has identified a promising therapeutic approach involving the inhibition of the ATP2B1 protein, a known regulator of cellular calcium (Ca2+) export and homeostasis. This discovery covered in this
COVID-19 News report, opens new avenues for reducing SARS-CoV-2 infection and replication, potentially offering a novel prophylactic treatment for COVID-19.
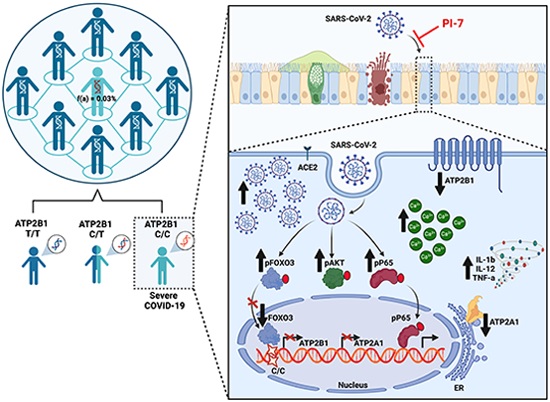 The ATP2B1 and ATP2A1 Ca2+ pumps are deregulated during SARS-CoV-2 infection, due to an impaired PI3K/Akt/FOXO3 pathway, which is antagonised by a small molecule promoting ATP2B1 activity. Moreover, a rare homozygous intronic ATP2B1 variant is associated with COVID19 severity.ATP2B1 (PMCA1) activation protects against SARS-CoV-2 infection.Loss of ATP2B1 increases SARS-CoV-2 viral replication. A nucleotide variant polymorphism (T > C) within the ATP2B1 (PMCA1) locus is associated with severe COVID19. A nontoxic caloxin-derived small molecule (PI-7) has potential for therapeutic applications against SARS‐CoV‐2.
The Role of Calcium in SARS-CoV-2 Replication
The ATP2B1 and ATP2A1 Ca2+ pumps are deregulated during SARS-CoV-2 infection, due to an impaired PI3K/Akt/FOXO3 pathway, which is antagonised by a small molecule promoting ATP2B1 activity. Moreover, a rare homozygous intronic ATP2B1 variant is associated with COVID19 severity.ATP2B1 (PMCA1) activation protects against SARS-CoV-2 infection.Loss of ATP2B1 increases SARS-CoV-2 viral replication. A nucleotide variant polymorphism (T > C) within the ATP2B1 (PMCA1) locus is associated with severe COVID19. A nontoxic caloxin-derived small molecule (PI-7) has potential for therapeutic applications against SARS‐CoV‐2.
The Role of Calcium in SARS-CoV-2 Replication
Calcium ions (Ca2+) play crucial roles in various cellular processes, including viral replication. Researchers have long recognized that many viruses, including SARS-CoV-2, exploit calcium signaling pathways to facilitate their life cycles. The ATP2B1 protein, a plasma membrane Ca2+ ATPase, is essential for maintaining low intracellular Ca2+ levels, which is crucial for cellular health and function.
In their study, the Naples team demonstrated that a nontoxic derivative of caloxin, termed PI-7, can effectively reduce intracellular Ca2+ levels, thereby impairing SARS-CoV-2 infection. By targeting ATP2B1, PI-7 helps to maintain Ca2+ homeostasis, which is disrupted during viral infection.
Unraveling the Mechanism: PI3K/Akt/FOXO Pathway
The mechanism behind ATP2B1's role in SARS-CoV-2 infection involves the PI3K/Akt signaling pathway. This pathway is crucial for various cellular functions, including metabolism, growth, and survival. Activation of the PI3K/Akt pathway leads to the inactivation of FOXO3, a transcription factor that regulates the expression of ATP2B1 and ATP2A1, another Ca2+ pump located in the endoplasmic reticulum.
During SARS-CoV-2 infection, the PI3K/Akt pathway becomes hyperactivated, leading to increased phosphorylation and inactivation of FOXO3. This results in reduced transcription of ATP2B1 and ATP2A1, causing a rise in intracellular Ca2+ levels, which the virus exploits to enhance its replication and propagation.
The Genetic Factor: A Rare ATP2B1 Variant
The study also uncovered a rare homozygous intronic variant o
f ATP2B1 that is associated with severe COVID-19. This variant, identified through genetic screening, suggests that individuals carrying this mutation might be at a higher risk of severe disease due to the dysregulation of Ca2+ homeostasis during infection.
PI-7: A Potential Game-Changer
Compound PI-7, derived from caloxin, emerges as a promising candidate for COVID-19 therapy. The research showed that PI-7 not only promotes ATP2B1 activity but also sustains the expression of both ATP2B1 and ATP2A1, thereby reducing the intracellular Ca2+ pool. This reduction hampers SARS-CoV-2 replication and propagation, offering a dual benefit: limiting viral spread and preventing severe disease outcomes.
Moreover, PI-7 has shown no toxicity in vitro, making it a viable candidate for further development as a prophylactic agent against COVID-19. The compound's ability to restore Ca2+ homeostasis and inhibit viral replication positions it as a potential therapeutic breakthrough.
Implications and Future Directions
The discovery of PI-7's effectiveness in targeting ATP2B1 and ATP2A1 to control intracellular Ca2+ levels present a new therapeutic strategy against SARS-CoV-2. This approach diverges from traditional antiviral treatments that target the virus directly, focusing instead on modulating host cellular pathways to impede viral replication.
Further research is needed to explore the full potential of PI-7, including clinical trials to assess its efficacy and safety in humans. Additionally, understanding the broader implications of ATP2B1 and ATP2A1 regulation in other viral infections could pave the way for new treatments for various Ca2+-dependent viruses.
Conclusion
The innovative work by the Naples research team highlights the importance of calcium signaling in viral infections and offers a novel therapeutic target in ATP2B1. By leveraging the nontoxic properties of PI-7 to maintain Ca2+ homeostasis, this approach holds promise for reducing SARS-CoV-2 infection and replication, potentially transforming the fight against COVID-19 and future pandemics.
The potential of PI-7 to serve as a prophylactic treatment underscores the need for continued research into host-pathogen interactions and the development of therapies that harness our understanding of cellular mechanisms to combat viral diseases. As we navigate the complexities of COVID-19, discoveries like these offer hope for more effective and innovative treatments in the near future.
The study findings were published in the peer reviewed journal: EMBO Reports.
https://www.embopress.org/doi/full/10.1038/s44319-024-00164-z
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/study-discovers-the-impact-of-sars-cov-2-e-and-m-proteins-on-host-cell-calcium-homeostasis-and-er-mitochondria-interactions
https://www.thailandmedical.news/news/austrian-study-shows-calcium-channel-inhibitors-including-amlodipine,-nifedipine,-felodipine-and-the-phytochemical-neferine-can-treat-covid-19-infecti
https://www.thailandmedical.news/news/breaking-cornell-study-find-that-entry-of-coronaviruses-is-a-ca2-dependent-process-calcium-channel-blockers-could-be-potential-covid-19-drugs
