Austrian Scientists Claim That High-Density Lipoproteins Can Be Used As Antivirals Against COVID-19 Infections
Nikhil Prasad Fact checked by:Thailand Medical News Team Mar 05, 2024 1 year, 9 months, 3 weeks, 6 days, 10 hours, 13 minutes ago
COVID-19 News: The ongoing battle against the global COVID-19 pandemic has led researchers to explore unconventional avenues for therapeutic intervention. In a recent development, scientists at the Medical University of Graz in Austria have unearthed compelling evidence suggesting that High-Density Lipoproteins (HDLs) could serve as potent antivirals against SARS-CoV-2 infections. This
COVID-19 News report delves into the intricate mechanisms underlying this discovery and explores the implications for future antiviral therapies.
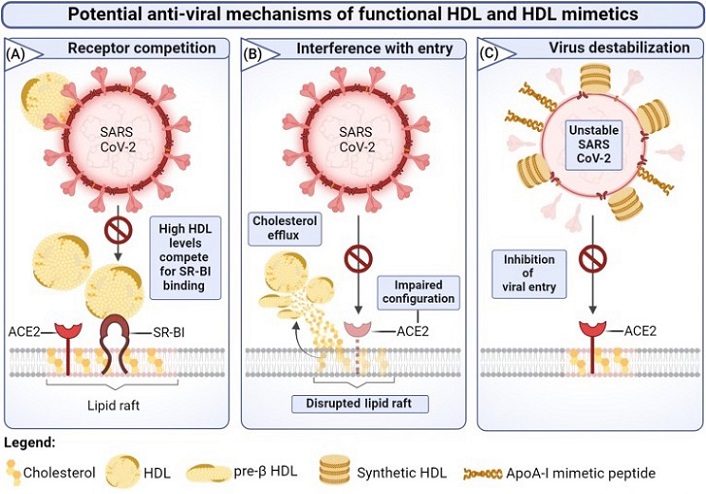 Potential antiviral effects of high-density lipoprotein (HDL) and HDL mimetics in coronavirus disease 2019.
(A) At high HDL particle concentrations, HDL competes with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/HDL complexes for scavenger receptor B type I (SR-BI) binding, thereby modulating the uptake of virus particles through the SR-BI–angiotensin-converting enzyme 2 (ACE2) axis. (B) HDL-mediated cholesterol depletion from lipid rafts disrupts the spatial organization of ACE2, potentially hindering SARS-CoV-2 attachment and subsequent cell entry. (C) Destabilization of virus particles by synthetic HDL mimetics prevents ACE2-mediated viral entry.
Multifunctionality of HDLs
Potential antiviral effects of high-density lipoprotein (HDL) and HDL mimetics in coronavirus disease 2019.
(A) At high HDL particle concentrations, HDL competes with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/HDL complexes for scavenger receptor B type I (SR-BI) binding, thereby modulating the uptake of virus particles through the SR-BI–angiotensin-converting enzyme 2 (ACE2) axis. (B) HDL-mediated cholesterol depletion from lipid rafts disrupts the spatial organization of ACE2, potentially hindering SARS-CoV-2 attachment and subsequent cell entry. (C) Destabilization of virus particles by synthetic HDL mimetics prevents ACE2-mediated viral entry.
Multifunctionality of HDLs
HDLs are intricate particles with multifaceted roles within the body's defense system. Evolving as a crucial component of innate immunity, recent research posits HDLs as pivotal in influencing susceptibility to SARS-CoV-2 infection and the severity of associated complications. Beyond their well-established role in cardiovascular health, HDLs emerge as dynamic lipoproteins impacting host defense against infections and injuries.
Cholesterol Extraction and Immune Enhancement
At the forefront of HDL's immune-modulating capabilities is its ability to extract cholesterol from cell membranes, influencing the arrangement and function of critical receptors involved in pathogen recognition. Acting as a scavenger, HDL also directly suppresses the immune response triggered by bacterial cell wall components and toxins. The multifunctionality of HDL is further underscored by over 250 associated proteins, contributing to innate immunity, inflammation, cellular adhesion, hemostasis, and protease regulation.
Synthetic HDLs in Clinical Trials
Exploiting the protective properties of HDL, scientists have developed synthetic and reconstituted HDL particles. For instance, CSL112, a novel formulation of human plasma-derived apolipoprotein A-I (ApoA-I), is currently undergoing evaluation in a phase 3 clinical trial. This innovation opens new avenues for harnessing HDL's anti-inflammatory effects in cardiometabolic diseases.
ACE2 Receptor and Cholesterol Efflux
Elevated HDL levels emerge as a potential shield against SARS-CoV-2 infection. The virus primarily ut
ilizes the angiotensin-converting enzyme 2 (ACE2) receptor for cellular entry. HDL's role in promoting cholesterol efflux may influence ACE2 receptor translocation to lipid rafts, affecting virus-cell interactions. Intriguingly, HDL serves as a bridge between the virus and scavenger receptor B type I (SR-BI), a crucial player in both cholesterol homeostasis and SARS-CoV-2 entry.
Competition for SR-BI Binding
Studies reveal that at high concentrations, HDL competes with SARS-CoV-2/HDL complexes for binding to SR-BI, suggesting that increasing HDL particles could be a therapeutic strategy to reduce viral infectivity. This novel insight presents a potential avenue for developing antiviral strategies that capitalize on HDL's inherent properties.
Inflammatory Impact on HDL
Chronic inflammation, aging, and cardiometabolic diseases often alter HDL composition, metabolism, and function. Individuals with COVID-19 exhibit reduced HDL levels and significant compositional changes, including elevated levels of serum amyloid A (SAA) and pulmonary surfactant-associated protein B. Diabetes-related glycation further impairs HDL function, contributing to exacerbated severity in COVID-19 patients.
HDl Dysfunction in COVID-19
Studies reveal reduced HDL ability to maintain endothelial barrier function and vascular health in SARS-CoV-2-infected individuals. Low HDL cholesterol efflux capacity and diminished levels of HDL-associated ApoM emerge as predictors of a fatal course in COVID-19 progression. Interaction between the SARS-CoV-2 spike protein and HDL raises questions about the impact on HDL's structure and function, necessitating further investigation.
Recombinant HDL Therapeutics
Drawing from anti-inflammatory properties observed in recombinant HDL therapeutics for atherosclerosis, researchers explore their potential as antiviral agents. CER-001, closely resembling natural HDL, has shown promise in reducing inflammatory cytokines and restoring a functional HDL profile. Synthetic HDL mimics, including the dimeric 4F peptide, exhibit antiviral potential by destabilizing viral particles.
Potential for Antiviral Therapeutics
Advancements in synthetic and reconstituted HDL production highlight the potential of HDL-based drug design for targeting SARS-CoV-2 infection. Fine-tuning nanoparticle composition with antiviral drugs, antioxidants, and therapeutic payloads opens new avenues for innovative antiviral therapeutics.
Conclusion
Austrian scientists' groundbreaking research sheds light on the unexplored territory of HDLs as potential antivirals against COVID-19. The intricate interplay between HDL particles, SARS-CoV-2, and host cells unveils promising avenues for therapeutic intervention. As research progresses, the prospect of HDL-based antiviral therapies brings hope for innovative solutions in the ongoing fight against the global pandemic.
The study findings were published in the peer reviewed journal: Trends in Molecular Medicine.
https://www.cell.com/trends/molecular-medicine/fulltext/S1471-4914(24)00026-1
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
