BREAKING COVID-19 News! First Study To Actually Show And Validate That SARS-CoV-2 Can Infect Human iPS Cell-Derived Sensory Neurons!
Nikhil Prasad Fact checked by:Thailand Medical News Team Sep 11, 2023 1 year, 7 months, 1 week, 14 hours, 57 minutes ago
COVID-19 News: The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has been an unprecedented global health crisis since its emergence in late 2019. The virus has affected billions of lives, causing widespread illness and death. As scientists and researchers continue to study this novel coronavirus, new findings are continually emerging, shedding light on its intricate interactions with the human body.
 Graphical Abstract
Graphical Abstract
A recent study conducted at the Whitehead Institute for Biomedical Research in Cambridge, USA, has uncovered a fascinating revelation: human sensory neurons can indeed be infected by SARS-CoV-2, marking an important milestone in our understanding of the virus's impact on the peripheral nervous system.
It should be noted that in previous studies, researchers were unable to conform or validate that the SARS-CoV-2 was able to infect the sensory neurons but did find other components of the CNS and neurological system were vulnerable to the SARS-CoV-2 virus. This would be the first study to actually demonstrate the actual infections of the sensory neurons by the SARS-CoV-2 virus!
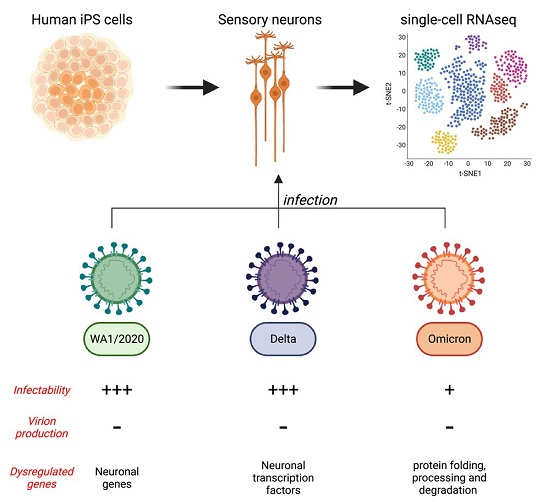
The study's findings reveal a comprehensive analysis of the infection of human sensory neurons by various strains of SARS-CoV-2, including the original WA1/2020 strain, as well as the delta and omicron variants. This groundbreaking research utilizes cutting-edge techniques, such as single-cell RNA sequencing (scRNA-seq) and immunofluorescence, to provide deep insights into the behavior of the virus within sensory neurons.
The Significance of Understanding SARS-CoV-2's Impact on the Nervous System
Before delving into the study's findings, it is essential to recognize the broader context of this research. COVID-19 is not merely a respiratory illness; it has also been associated with a range of neurological symptoms, including loss of taste and smell, headaches, stroke, delirium, and brain inflammation as covered in previous studies and also
COVID-19 News reports. One of the earliest and most distinctive neurological symptoms observed in COVID-19 patients is anosmia, the loss of smell. While most patients recover their senses of smell and taste within a few weeks, some individuals continue to experience persistent or poorly recovered anosmia.
Recent studies have suggested that SARS-CoV-2 can disrupt the nuclear architecture of the olfactory epithelium, leading to the dysregulation of olfactory receptor genes. Additionally, other peripheral neuropathies, such as small-fiber neuropathy, multifocal demyelinating neuropathy, and critical illness axonal neuropathy, have been identified in COVID-19 patients. These neuropathies often manifest at later stages of the disease, when the initial inflammatory response
has subsided.
Given these observations, understanding how SARS-CoV-2 interacts with the peripheral nervous system, specifically sensory neurons, is crucial. The study at the Whitehead Institute set out to investigate whether human sensory neurons could be infected by the virus and to what extent different strains, including variants, can infect these cells.
Human Sensory Neurons Express ACE2 Receptor
To explore the potential infection of human sensory neurons by SARS-CoV-2, the researchers first assessed whether these neurons express the ACE2 receptor, which is the key protein allowing the virus to enter host cells. Cells lacking ACE2 receptors are generally resistant to infection by SARS-CoV-2. Using induced pluripotent stem cells (iPS cells), the researchers successfully generated human sensory neurons and found that over 90% of these cells expressed sensory neuron markers.
Utilizing single-cell RNA sequencing (scRNA-seq), the scientists further analyzed the population of sensory neurons and identified two distinct clusters. Cluster 1 contained cells expressing high levels of Doublecortin (DCX), which is present in immature neurons and adult dorsal root ganglia. Clusters 2 and 3 consisted of cells expressing high levels of sensory neuron markers NTRK2 and NTRK3.
Importantly, ACE2 expression was similar in clusters 2 and 3, indicating that sensory neurons indeed express the ACE2 receptor, making them susceptible to SARS-CoV-2 infection.
Infection of Human Sensory Neurons by SARS-CoV-2
With evidence of ACE2 expression in human sensory neurons, the researchers proceeded to investigate whether these neurons could be infected by SARS-CoV-2. They conducted scRNA-seq on sensory neurons exposed to both heat-inactivated (HI) and fully active WA1/2020 SARS-CoV-2. For comparison, HEK293T (293T) cells, which express ACE2 and actively replicate the virus, were included in the study.
The results were striking. Approximately 30% of the sensory neurons exposed to live SARS-CoV-2 were found to have viral RNA, as indicated by the presence of Nucleocapsid RNA. Importantly, sensory neurons positive for SARS-CoV-2 RNA exhibited reduced expression of genes related to RNA metabolism, including FUS, NUCKS1, and NCL. These genes are known to be involved in RNA processing and transport, suggesting that SARS-CoV-2 infection may impact sensory neurons' RNA metabolism. This alteration in RNA metabolism may play a role in inhibiting viral RNA translation within these cells.
Sensory Neurons Do Not Produce Infectious SARS-CoV-2 Virions
One of the most intriguing aspects of this study is the discovery that although human sensory neurons can be infected by SARS-CoV-2, they do not produce infectious virus particles. This revelation was confirmed through multiple assays, including plaque assays and immunofluorescence detection of viral double-stranded RNA (dsRNA).
The SARS-CoV-2 virus primarily consists of positive-sense, single-stranded RNA. Upon entering host cells, it uses the host cell's machinery to generate a complementary negative-sense RNA, which serves as a template for producing additional positive-sense viral genomes. These positive-sense genomes are essential for viral protein translation and are incorporated into newly formed virions.
The presence of negative-sense SARS-CoV-2 RNA is indicative of active viral RNA replication. ScRNA-seq data revealed that negative strands of SARS-CoV-2 RNA were detectable in sensory neurons exposed to the WA1/2020 strain and the delta variant, but not the omicron variant. This suggests that viral RNA synthesis occurs in sensory neurons infected by specific strains of SARS-CoV-2.
Plaque assays further confirmed that sensory neurons do not shed infectious SARS-CoV-2 virions, as opposed to control cells like Calu-3 lung carcinoma cells, which demonstrated robust viral replication. Immunofluorescence staining revealed the presence of viral proteins, including Nucleocapsid, in infected sensory neurons, providing additional evidence of viral activity within these cells.
The mechanism by which sensory neurons block the assembly and release of progeny virions remains an intriguing question that warrants further investigation.
Infection by SARS-CoV-2 Variants Delta and Omicron
The study also explored the infectivity of sensory neurons by different SARS-CoV-2 variants, including the delta and omicron variants. Peripheral neuropathies, including anosmia, have been reported in COVID-19 patients. The delta variant seemed to affect the peripheral nervous system similarly to the original WA1/2020 strain. However, the omicron variant, known for its increased transmissibility and milder symptoms, showed a decrease in reported anosmia and peripheral neuropathies.
The research involved infecting co-cultures of iPS-derived sensory neurons and 293T cells with the three variants - WA1/2020, delta, and omicron. The findings demonstrated that all three variants had the ability to infect both sensory neurons and 293T cells. However, the delta variant showed a higher infectivity rate in 293T cells compared to the WA1/2020 strain but a lower rate in sensory neurons.
Conversely, the omicron variant displayed significantly lower infectivity rates in both sensory neurons and 293T cells compared to the other two variants.
Interestingly, the gene expression profiles of infected sensory neurons and 293T cells exhibited variant-specific patterns. For instance, ZEB1 was upregulated exclusively upon WA1/2020 and delta infection of sensory neurons. This gene plays a role in repressing ACE2 expression and is related to epithelial-mesenchymal transition (EMT). CTNNA2, crucial for neural fitness, was downregulated upon WA1/2020 and delta infection of sensory neurons. BMI1, linked to senescence, was upregulated in response to all three variants in 293T cells. STIM1, which promotes SARS-CoV-2 infection, exhibited varying expression patterns among the variants. Additionally, the omicron variant induced a unique transcriptomic signature in both sensory neurons and 293T cells.
Implications and Future Directions
This study conducted at the Whitehead Institute for Biomedical Research provides valuable insights into the interaction between SARS-CoV-2 and human sensory neurons. It challenges previous assumptions that neurons are resistant to infection due to a lack of ACE2 expression. Instead, this research demonstrates that a significant fraction of sensory neurons can be infected, potentially shedding light on the neuropathies observed in COVID-19 patients.
The findings also raise intriguing questions about the mechanisms by which sensory neurons inhibit the production of infectious virions, despite harboring viral RNA and protein. Further exploration of the phosphorylation landscape and the role of tyrosine kinases in infected sensory neurons may yield critical insights into this phenomenon.
Moreover, the study highlights the differences in infectivity and gene expression profiles among SARS-CoV-2 variants. The omicron variant, despite being less severe, exhibited unique characteristics compared to the original strain and the delta variant. Understanding these variant-specific interactions with sensory neurons may contribute to our comprehension of the diverse clinical manifestations of COVID-19.
The study findings were published in the peer reviewed journal: iScience.
https://www.cell.com/iscience/fulltext/S2589-0042(23)01767-4
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
