BREAKING COVID-19 News! Integrin α5β1 Contributes To Cell Fusion And Inflammation Mediated By SARS-Cov-2 Spike Via RGD-Independent Interaction!
Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 11, 2023 1 year, 4 months, 1 week, 2 days, 3 hours, 1 minute ago
COVID-19 News: The COVID-19 pandemic, caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has presented unprecedented challenges to global health. Understanding the intricate interactions between the virus and host cells is crucial for developing effective therapeutic strategies. Recent research covered in this
COVID-19 News report, conducted at the Versiti Blood Research Institute in Milwaukee, USA, has shed light on the role of Integrin α5β1 in SARS-CoV-2 infection, revealing its contribution to cell fusion and inflammation via a unique RGD-independent mechanism.
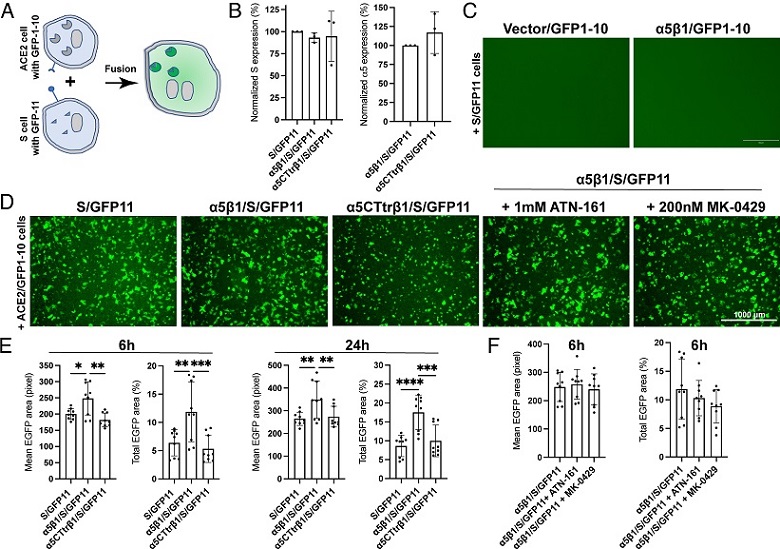 Integrin α5β1 contributes to cell–cell fusion mediated by SARS-CoV-2 spike. (A) Diagram of cell–cell fusion assay measured by split GFP assay. (B) Surface expression of SARS2-S and α5 in HEK293T-α5-KO cells. Cells were transfected with indicated constructs for 48 h and subjected to flow cytometry before the cell–cell fusion assay. Data are mean ± SD from four independent repeats. (C) α5β1 alone in the absence of ACE2 does not mediate cell–cell fusion induced by SARS2-S. Representative images of three independent repeats. (D) Representative images of HEK293T-α5-KO cells transfected with ACE2 plus GFP1-10 cocultured for 6 h with HEK293T-α5-KO cells transfected with S/GFP11, α5β1/S/GFP11, or α5CTtrβ1/S/GFP11. The α5β1/S/GFP11 cells were also pretreated with ATN-161 or MK-0429 before coculturing with the ACE2/GFP1-10 cells. Cells were imaged using AMG EVOS fluorescence microscope with Plan Fluor 4× objective lens (numerical aperture of 0.13), equipped with Sony 1cx285AQ color CCD camera. (E and F) Quantification of cell–cell fusion at 6 h and 24 h after coculturing. Three images were randomly taken for each experiment group in three independent repeats. The mean or total EGFP area was measured using CellProfiler software. Data are mean ± SD. One-Way ANOVA Tukey’s multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
The Landscape of COVID-19 Pathogenesis
Integrin α5β1 contributes to cell–cell fusion mediated by SARS-CoV-2 spike. (A) Diagram of cell–cell fusion assay measured by split GFP assay. (B) Surface expression of SARS2-S and α5 in HEK293T-α5-KO cells. Cells were transfected with indicated constructs for 48 h and subjected to flow cytometry before the cell–cell fusion assay. Data are mean ± SD from four independent repeats. (C) α5β1 alone in the absence of ACE2 does not mediate cell–cell fusion induced by SARS2-S. Representative images of three independent repeats. (D) Representative images of HEK293T-α5-KO cells transfected with ACE2 plus GFP1-10 cocultured for 6 h with HEK293T-α5-KO cells transfected with S/GFP11, α5β1/S/GFP11, or α5CTtrβ1/S/GFP11. The α5β1/S/GFP11 cells were also pretreated with ATN-161 or MK-0429 before coculturing with the ACE2/GFP1-10 cells. Cells were imaged using AMG EVOS fluorescence microscope with Plan Fluor 4× objective lens (numerical aperture of 0.13), equipped with Sony 1cx285AQ color CCD camera. (E and F) Quantification of cell–cell fusion at 6 h and 24 h after coculturing. Three images were randomly taken for each experiment group in three independent repeats. The mean or total EGFP area was measured using CellProfiler software. Data are mean ± SD. One-Way ANOVA Tukey’s multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
The Landscape of COVID-19 Pathogenesis
SARS-CoV-2 utilizes the angiotensin-converting enzyme 2 (ACE2) receptor for cell entry, leading to the development of COVID-19. The complexity of the disease, affecting multiple organs and eliciting dysregulated inflammatory responses, suggests involvement of additional cellular factors beyond ACE2. Integrin α5β1, a cell adhesion molecule found in various tissues, has emerged as one such factor.
The study explored the exploitation of α5β1 signaling by the SARS-CoV-2 spike protein, shedding light on its contribution to cell–cell fusion and inflammation, potentially influencing the infection and pathogenesis of COVID-19.
Exploring the Role of Integrin α5β1 in SARS-CoV-2 Infection
The research team investigated the interaction between SARS-CoV-2 spike (S) protein and integrin &
amp;alpha;5β1, focusing on its potential involvement in virus entry and cell–cell fusion. While ACE2 serves as the primary receptor for SARS-CoV-2 entry, the study revealed that α5β1 does not directly contribute to virus cell entry.
However, it was found to enhance S-mediated cell–cell fusion in collaboration with ACE2. Strikingly, this effect was not inhibited by known α5β1 inhibitors or RGD-mimetic inhibitors, suggesting a non-traditional mechanism.
Understanding the Molecular Basis of SARS-CoV-2 and α5β1 Interaction
The investigation delved into the molecular basis of the interaction between α5β1 and the S protein. Contrary to expectations, the interaction was found to be independent of the RGD-containing receptor binding domain. Instead, it involved the S2 subunit of the S protein and α5β1 homo-oligomerization. The study provided insights into the unique nature of the interaction, challenging previous models and emphasizing the need for further structural studies.
Contribution to Inflammatory Responses
Beyond its role in cell–cell fusion, the S protein was observed to induce inflammatory responses in human endothelial cells. These responses were characterized by NF-κB activation, gasdermin D cleavage, and increased secretion of proinflammatory cytokines IL-6 and IL-1β. Notably, the loss of α5 expression or inhibition of the α5 cytoplasmic tail binding protein phosphodiesterase-4D (PDE4D) attenuated these effects, indicating the involvement of α5β1 in SARS-CoV-2-induced inflammation. This finding opens avenues for potential antiviral treatments targeting the α5β1 pathway.
Key Findings:
The research unveiled several key findings:
-Integrin α5β1 does not play a direct role in SARS-CoV-2 cell entry but enhances S-mediated cell–cell fusion.
-The interaction between α5β1 and S protein is independent of the RGD motif and involves the S2 subunit and α5β1 homo-oligomerization.
-SARS-CoV-2-induced inflammatory responses in endothelial cells are mediated by α5β1 through the PDE4D pathway.
-The inflammatory cascade involves NF-κB activation, cytokine release, and gasdermin D cleavage.
Challenges and Controversies in Integrin Research
The involvement of integrins in SARS-CoV-2 infection has been a subject of debate. With 24 α/β integrin heterodimers in humans, integrins play critical roles in various biological processes. Previous studies suggested a potential role for integrins, particularly α5β1, based on the presence of an RGD motif on the S protein. However, conflicting findings and controversies surrounded the RGD-dependent interaction. This study findings add to this discourse, proposing a nonclassical RGD-independent ligand-binding and signaling function of integrin α5β1 in the context of SARS-CoV-2 infection.
Implications for COVID-19 Pathogenesis
The severity of COVID-19 is associated with dysregulated inflammatory responses, and the study suggests that α5β1-mediated cell–cell fusion and inflammation contribute to the complex pathogenesis of SARS-CoV-2. While the exact host receptors responsible for COVID-19 pathogenesis are not fully delineated, the study provides molecular insights into the role of α5β1 and offers potential targets for antiviral treatments.
Conclusion: Targeting α5β1 for Therapeutic Intervention
The study at Versiti Blood Research Institute significantly contributes to our understanding of SARS-CoV-2 pathogenesis, uncovering the multifaceted role of integrin α5β1 in cell fusion and inflammation. These findings not only challenge existing models but also propose potential targets for antiviral treatments. Inhibiting the α5β1 pathway could offer a novel therapeutic approach to mitigate the severity of COVID-19 and reduce inflammatory responses associated with SARS-CoV-2 infection. As research continues to unravel the complexities of virus-host interactions, targeting specific cellular factors like α5β1 may pave the way for innovative strategies in the battle against COVID-19.
The study findings were published in the peer reviewed journal: PNAS.
https://www.pnas.org/doi/10.1073/pnas.2311913120
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
