BREAKING! COVID-19 News: Italian Researchers Warn That SARS-CoV-2 Can Infect The Thymus And Impair T Cell Generation!
COVID-19 News - SARS-CoV-2 - Thymus - T cells Feb 18, 2023 2 years, 2 months, 3 hours, 15 minutes ago
COVID-19 News: A new study by Italian researchers has found that the SARS-CoV-2 virus is able to infect the thymus and impair T cell generation that can lead to disease severity.
The fact that the SARS-CoV-2 can infect the thymus also leads to the possibility of long-term immune dysfunction or immunodeficiency issues!
Already in our past
COVID-19 News coverages, we did cover studies that showed that the thymus was also vulnerable to the SARS-CoV-2 virus.
https://www.thailandmedical.news/news/breaking-sars-cov-2-causes-thymic-dysregulation-and-thymic-atrophy-that-results-in-lymphopenia-and-peripheral-t-cell-receptor-repertoire-changes
This new Italian study however validates that the thymus gland is indeed susceptible to infection and damage by the SARS-CoV-2 virus as it also contains the ACE-2 receptors.
The study team comprised of researchers from:
-Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
-Sapienza University of Rome, Rome, Italy
-INMI L Spallanzani – IRCCS, Rome, Italy
-University of Rome Tor Vergata, Rome, Italy
-University of Naples Federico II, Rome, Italy
-Catholic University of the Sacred Heart, Rome, Italy
Already, lymphopenia, particularly when restricted to the T-cell compartment, has been described as one of the major clinical hallmarks in patients with coronavirus disease 2019 (COVID-19) and proposed as an indicator of disease severity.
Though several mechanisms fostering COVID-19–related lymphopenia have been described, including cell apoptosis and tissue homing, the underlying causes of the decline in T-cell count and function are still not completely understood.
Considering that viral infections can directly target thymic microenvironment and impair the process of T-cell generation, the study team sought to investigate the impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on thymic function.
The researchers performed molecular quantification of T-cell receptor excision circles and κ-deleting recombination excision circles to assess, respectively, T- and B-cell neogenesis in SARS-CoV-2–infected patients.
The study team also developed a system for in vitro culture of primary human thymic epithelial cells (TECs) to mechanistically investigate the impact of SARS-CoV-2 on TEC function.
The study findings showed that patients with COVID-19 had reduced thymic function that was inversely associated with the severity of the disease.
Importantly, the study findings revealed that angiotensin-converting enzyme 2 (ACE-2), through which SARS-CoV-2 enters the host cells, was expressed by thymic epithelium, and in particular by medullary TECs.
The study findings also showed that SARS-CoV-2 can target TECs and downregulate critical genes and pathways associated with epithelial cell adhesion and survival.
The study findings demonstrated that the human thymus is a target of SARS-CoV-2 and thymic function is altered following infection.
The study findings expand the current knowledge of the effects of SARS-CoV-2 infection on T-cell homeostasis and suggest that monitoring thymic activity may be a useful marker to predict disease severity and progression.
the study findings were published in the peer reviewed Journal of Allergy and Clinical Immunology.
https://www.sciencedirect.com/science/article/pii/S0091674923001471
Despite a large number of studies that have investigated the effects of the infection on lymphopenia, very few have assessed whether the process of T-cell and B-cell lymphopoiesis is altered in patients with COVID-19.
This is the first study to present for the first-time evidence that the thymus is a target of SARS-CoV-2 infection in vivo and that thymic function is impaired in patients with COVID-19.
The study team first demonstrated that patients with COVID-19 display decreased levels of TRECs and KRECs, which represent surrogate markers of T- and B-cell neogenesis, respectively.
The study data demonstrated a more severe reduction in TREC levels in both non-ICU and ICU patients, whereas KRECs were less affected.
Considering these findings, the study team sought to investigate a possible direct impact of SARS-CoV-2 on the thymus.
The study findings demonstrated that thymic epithelium of pediatric and adult patients expresses the SARS-CoV-2 receptor ACE2 and that SARS-CoV-2 entry into the cells resulted in fundamental changes in gene expression profile.
The study data suggest that following infection, SARS-CoV-2 recruits a variety of host factors to survive and propagate itself, as suggested by the activation of different pathways associated with ribosome biogenesis and protein translation. These effects are associated with the derangement of the normal gene expression profile in TECs, leading to the downregulation of crucial pathways involved in epithelium cell maintenance (such as focal adhesion and extracellular matrix interaction) and upregulation of metabolic processes (such as oxidative phosphorylation).
It was noted that the activation of these metabolic pathways may result in the rise of oxidative stress, which could explain the decrease in vitality and increase in mitophagy pathway observed in SARS-CoV-2–infected cells.
Though the study data showed that primary hTECs were unable to sustain effective viral replication in an in vitro culture system, the findings demonstrated that SARS-CoV-2 can persist in infected hTECs over time in vitro and in vivo.
Hence, along with the changes induced by the direct infection of cells, recirculating virus-specific T cells could target infected TECs and significantly contribute to thymic damage and reduced thymic output.
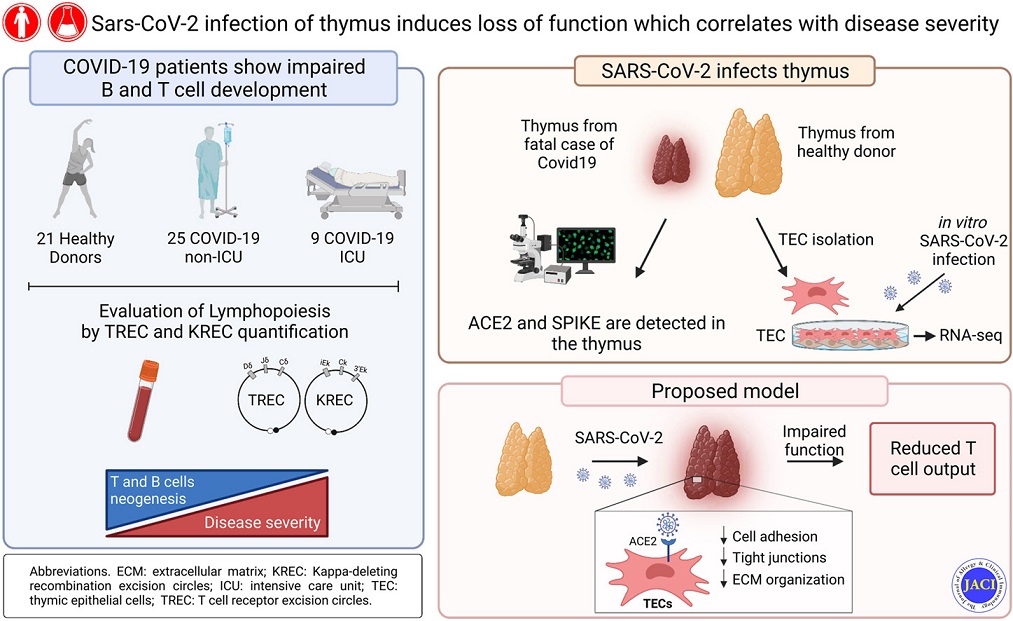 Graphical Abstract
Graphical Abstract
Past studies conducted on mouse models of mycobacterial and lymphocytic choriomeningitis virus (LCMV) infections support this hypothesis.
https://www.pnas.org/doi/abs/10.1073/pnas.1913776117
https://journals.aai.org/jimmunol/article/190/4/1646/39630
Importantly, impaired thymic function in patients with COVID-19 could potentially lead to significant immunologic consequences.
Besides its vital importance in generating the T-cell pool during early life, optimal thymic function is required to reestablish T-cell immunity after periods of immunologic insults, such as those caused by antineoplastic therapies, immunosuppressive treatments, and infections.
Reduced thymic function and the resulting decrease in T-cell export could exacerbate lymphopenia in acutely ill patients with COVID-19 and increase the time required to restore the number and function of circulating T-cells after recovery.
Worse, delayed recovery of thymic function may contribute to the development of secondary infections, which can worsen the severity of the illness, increase the risk of disease progression, and facilitate persistent symptoms associated with herpesvirus reactivation.
https://www.mdpi.com/2076-0817/10/6/763?trk=public_post_comment-text
Also, the direct impact of SARS-CoV-2 on TECs and the additional damage caused by the rise in proinflammatory molecules, such as interferons, IL-6, and TNF-α, which have all been implicated in acute thymic involution, could lead to suboptimal education of the developing thymocytes.
https://link.springer.com/article/10.1007/s00005-017-0462-x
Worryingly, an impaired process of T-cell development might alter the process of central tolerance and generate the maturation of T-cell responses to self-antigens that lead to the development of autoimmune response against self-tissue antigens.
Already, the relationship between viral infections and the development of autoimmune diseases, such as multiple sclerosis, rheumatoid arthritis, type 1 diabetes, and SLE, is well known.
https://www.mdpi.com/1999-4915/11/8/762
The underlying mechanisms of this association are still largely unexplored, although evidence suggests that molecular mimicry, bystander T-cell activation, and abnormal nucleic acid sensing may play a role. Breaching of central tolerance has been also proposed. Several viruses, including HIV, measles virus, LCMV, yellow fever virus, and Zika virus, can cause thymic involution and altered thymic epithelium architecture and function.
A recent study involving mouse models of roseolovirus and LCMV infections demonstrated a direct link between viral infection and the development of autoimmunity and self-reactive T cells.
https://rupress.org/jem/article/219/3/e20211403/213039/Disruption-of-thymic-central-tolerance-by
https://www.pnas.org/doi/abs/10.1073/pnas.1913776117
COVID-19 generates a strong and excessive inflammatory response involving pathways and targets known to be commonly associated with autoimmune and inflammatory diseases. Whether SARS-CoV-2 induces or exacerbates autoimmunity is an active area of research, particularly now that several reports on a potential association of COVID-19 with autoimmune diseases (such as idiopathic thrombocytopenic purpura, multisystem inflammatory syndrome in children, autoimmune thyroid disease, and Guillain−Barré syndrome) have gradually started to emerge.
https://www.frontiersin.org/articles/10.3389/fimmu.2020.02055/full
Interestingly, studies in experimental mouse models have demonstrated that infections of the thymic microenvironment results in the emergence of pathogen-specific tolerance, as shown for LCMV, murine leukemia virus, hepatitis B virus, and Mycobacterium avium infections.
https://rupress.org/jem/article/172/6/1765/24760/Neonatal-exposure-to-thymotropic-gross-murine
https://journals.aai.org/jimmunol/article/184/1/351/81866
Whether this is relevant in human infections is a matter of investigation.
This is the first study to establish that SARS-CoV-2 can directly target the thymus and alter gene expression profile of thymic epithelium.
The study team proposes that the evaluation of thymic functionality (eg, by quantifying sjTRECs in patient peripheral blood) may be a useful marker to identify patients with COVID-19 at risk of complication both in acute and convalescent phases.
The study data on altered expression of potential autoantigens raises the possibility that disruption of thymic function by SARS-CoV-2 may be an additional mechanism to explain the excessive inflammation and potentially contribute to the development of autoimmune diseases related to COVID-19.
For the latest
COVID-19 News, keep on logging to Thailand Medical News.

