BREAKING COVID-19 News! SARS-CoV-2 Found To Also Use Human Transferrin Receptor For Viral Entry!
Nikhil Prasad Fact checked by:Thailand Medical News Team Feb 28, 2024 1 year, 1 month, 3 weeks, 5 days, 11 hours, 10 minutes ago
COVID-19 News: The ongoing battle against the COVID-19 pandemic has prompted relentless scientific exploration to comprehend the intricacies of the SARS-CoV-2 virus. Recent findings from the Kunming College of Life Science, University of Chinese Academy of Sciences, and the Chinese Academy of Sciences in Beijing and Kunming have unveiled a novel aspect of viral entry - the exploitation of the human transferrin receptor (TfR). This discovery sheds light on an alternative pathway for viral entry and infection, opening avenues for potential antiviral strategies. This
COVID-19 News report delves into the significance of this research, the characteristics of TfR, its role in SARS-CoV-2 infection, and the implications for future therapeutic interventions.
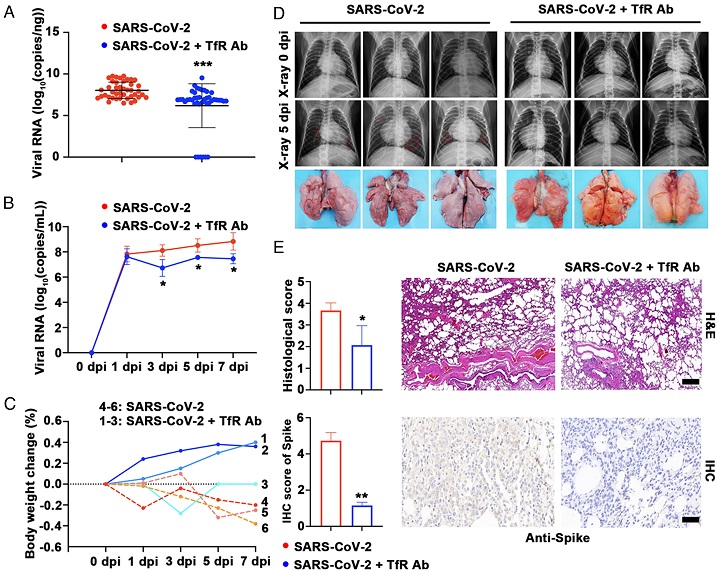 SARS-CoV-2 Found To Also Use Human Transferrin Receptor For Viral Entry. Anti-TfR antibody shows significant anti-COVID-19 effects in the rhesus macaque model. Monkeys were randomly assigned to saline (SARS-CoV-2, n = 3) or anti-TfR antibody-treated groups (SARS-CoV-2 + 1.6 mg/kg monkey TfR Ab, n = 3) and inoculated with SARS-CoV-2 via intratracheal and intranasal administration. (A) All seven lung lobes from all monkey groups were harvested for evaluation of viral loads at 7 dpi. No viral RNA was detected in six of the forty-two samples, two of them from the lower left lobe of monkey 3 and the remaining from the middle and lower right lobe of monkey 1. (B) Viral loads in respiratory epithelium. (C) The body weight change of every monkey was recorded, 1 to 3: SARS-CoV-2 infected group, 4 and 5: SARS-CoV-2 and TfR Ab treatment group. (D, Top) Radiographs of each animal taken on 0 and 5 dpi are shown. (D, Bottom) The pulmonary infiltration area is marked with a red circle. Photographs of lung specimens of all monkey groups at 7 dpi are shown. (E) Histopathological changes and IHC in lung sections of all monkey groups at 7 dpi were analyzed by H&E staining and anti-spike antibody staining. Corresponding quantification is shown on the Right. (Scale bar, 50 μm.) Data represent mean ± SD (n = 3), *P < 0.05, **P < 0.01, ***P < 0.001 by the unpaired t test (A and E) or two-way ANOVA and Fisher’s least significant difference (LSD) tests (B).
Understanding Human Transferrin Receptor (TfR)
SARS-CoV-2 Found To Also Use Human Transferrin Receptor For Viral Entry. Anti-TfR antibody shows significant anti-COVID-19 effects in the rhesus macaque model. Monkeys were randomly assigned to saline (SARS-CoV-2, n = 3) or anti-TfR antibody-treated groups (SARS-CoV-2 + 1.6 mg/kg monkey TfR Ab, n = 3) and inoculated with SARS-CoV-2 via intratracheal and intranasal administration. (A) All seven lung lobes from all monkey groups were harvested for evaluation of viral loads at 7 dpi. No viral RNA was detected in six of the forty-two samples, two of them from the lower left lobe of monkey 3 and the remaining from the middle and lower right lobe of monkey 1. (B) Viral loads in respiratory epithelium. (C) The body weight change of every monkey was recorded, 1 to 3: SARS-CoV-2 infected group, 4 and 5: SARS-CoV-2 and TfR Ab treatment group. (D, Top) Radiographs of each animal taken on 0 and 5 dpi are shown. (D, Bottom) The pulmonary infiltration area is marked with a red circle. Photographs of lung specimens of all monkey groups at 7 dpi are shown. (E) Histopathological changes and IHC in lung sections of all monkey groups at 7 dpi were analyzed by H&E staining and anti-spike antibody staining. Corresponding quantification is shown on the Right. (Scale bar, 50 μm.) Data represent mean ± SD (n = 3), *P < 0.05, **P < 0.01, ***P < 0.001 by the unpaired t test (A and E) or two-way ANOVA and Fisher’s least significant difference (LSD) tests (B).
Understanding Human Transferrin Receptor (TfR)
The human transferrin receptor is a vital carrier protein responsible for importing iron into cells, crucial for maintaining cellular iron homeostasis. Its expression levels vary across different cell types, with high levels observed in red blood cells, brain endothelial cells, and certain tumor cells. The regulation of TfR production is intricately linked to intracellular iron levels, ensuring the proper balance necessary for cellular functions. Iron-responsive element-binding proteins IRP1 and IRP2 regulate TfR production in response to iron levels, stabilizing TfR mRNA in the absence of iron.
The human transferrin receptor (TfR) serves as a pivotal player in cellular iron
uptake, a process vital for various physiological functions ranging from oxygen transport to DNA synthesis. TfR's significance lies in its role as a carrier protein for transferrin, the primary iron-binding protein in circulation. By facilitating the internalization of the transferrin-iron complex through receptor-mediated endocytosis, TfR regulates intracellular iron levels in response to the cell's metabolic demands.
TfR exists in two main isoforms, TfR1 and TfR2, with distinct expression patterns and regulatory mechanisms. TfR1, characterized by high affinity and ubiquitous expression, is essential for iron acquisition by most cells, particularly developing erythrocytes. In contrast, TfR2 expression is more restricted and less influenced by intracellular iron concentrations. While TfR1 predominantly mediates iron uptake, several studies suggest the existence of alternative mechanisms for transferrin uptake, hinting at the complexity of cellular iron homeostasis.
Moreover, TfR expression levels are tightly regulated in response to intracellular iron levels by iron-responsive element-binding proteins (IRPs). In conditions of low iron availability, IRPs bind to specific iron-responsive elements (IREs) present in the 3' untranslated region (UTR) of TfR mRNA, stabilizing its transcript and enhancing translation. This regulatory mechanism ensures that cells increase TfR expression to enhance iron intake under conditions of iron deficiency, thereby maintaining cellular iron homeostasis.
Beyond its canonical role in iron uptake, emerging evidence suggests that TfR possesses additional functions beyond iron metabolism. Studies have highlighted TfR's involvement in various cellular processes, including cell proliferation, differentiation, and immune response modulation. Notably, TfR expression is elevated in rapidly dividing cells, such as tumor cells, and its upregulation is associated with increased cellular iron demand to support enhanced metabolic activity.
In the context of viral infections, TfR's multifaceted nature becomes particularly intriguing. While it primarily serves as a gateway for cellular iron acquisition, TfR has also been implicated as a receptor/co-receptor for several viruses, facilitating their entry into host cells. This dual role underscores TfR's versatility and underscores its potential as a target for therapeutic intervention against viral infections, including SARS-CoV-2.
SARS-CoV-2 Exploits TfR for Viral Entry
Despite the well-established role of angiotensin-converting enzyme-2 (ACE2) as the primary receptor for SARS-CoV-2, this study illuminates the presence of alternative receptors or co-receptors, given the virus's detection in organs with minimal or no ACE2 expression.
The researchers identified human TfR as a novel receptor for SARS-CoV-2, mediating viral entry by directly binding to the spike protein. This interaction was characterized by a high affinity, with a dissociation constant (KD) of approximately 2.95 nM. The study highlights that interference with the TfR-SARS-CoV-2 interaction significantly inhibits viral infection, indicating TfR as a potential target for antiviral strategies.
Expression and Regulation of TfR in Response to SARS-CoV-2
Analyzing TfR expression in the lungs, trachea, and nasal cavity of mice revealed higher levels in respiratory organs, correlating with the preferential infection of respiratory cells by SARS-CoV-2. Additionally, SARS-CoV-2 infection led to an up-regulation of TfR expression in the lungs of monkeys and humanized ACE2 mice, suggesting a potential role in the viral life cycle. This insight into the regulation of TfR by viral infection expands our understanding of the virus-host interaction.
Mechanism of TfR-Mediated SARS-CoV-2 Entry
The study elucidates the mechanism by which TfR facilitates SARS-CoV-2 entry. Through Co-immunoprecipitation (Co-IP) and electron microscopy, the researchers observed the physiological interaction between TfR and the SARS-CoV-2 spike protein. Further experiments demonstrated that TfR-mediated viral endocytosis occurs by direct interaction with the spike protein, implicating TfR as a crucial player in the viral entry process. Designed peptides and an anti-TfR antibody were found to effectively inhibit SARS-CoV-2 infection, paving the way for potential therapeutic interventions.
ACE2-Independent Role of TfR in SARS-CoV-2 Infection
Contrary to conventional wisdom, the study uncovered that TfR-mediated SARS-CoV-2 infection is independent of ACE2. ACE2 knockout only partially inhibited viral infection, while TfR knockdown and overexpression resulted in a significant reduction and increase in viral infection, respectively. The findings emphasize TfR's importance in viral infection, offering insights into a potential parallel entry pathway that could be targeted for therapeutic purposes.
TfR as a Susceptibility Factor and Therapeutic Target
The study extended its scope to mouse models, demonstrating that mice overexpressing human TfR become susceptible to SARS-CoV-2 infection. This finding reinforces the relevance of TfR as a potential susceptibility factor for the virus. The anti-TfR antibody exhibited significant antiviral effects in a rhesus macaque model, inhibiting viral replication and alleviating pneumonia. These promising results underscore TfR as a plausible therapeutic target for mitigating SARS-CoV-2 infections.
Implications for Antiviral Strategies and Future Research
The discovery of TfR as a receptor for SARS-CoV-2 broadens our understanding of viral entry mechanisms and presents new possibilities for antiviral strategies. The inhibitory effects of soluble TfR, designed peptides, and the anti-TfR antibody highlight potential avenues for drug development. The research also establishes an hTfR-expressing mouse model, providing a valuable tool for studying viral infections and screening potential drugs.
Conclusion
In unraveling the role of human transferrin receptor as a key player in SARS-CoV-2 entry, this groundbreaking study enhances our comprehension of the virus-host interaction. TfR's ACE2-independent mediation of viral infection and its susceptibility factor role in mouse models emphasize its significance in the context of COVID-19. The anti-TfR antibody's success in inhibiting viral replication in macaques signals a promising avenue for therapeutic development. As the world continues to combat the pandemic, this newfound understanding of alternative viral entry pathways may pave the way for innovative antiviral strategies and therapeutic interventions.
Thailand
Medical News would like to add that further studies are needed to assess if the original Wuhan wildtype strain or earlier SARS-CoV-2 variants had the same potential to bind to these Human Transferrin Receptors or is it an evolved trait that only is seen in all the Omicron and subsequent sub-lineages.
The study findings were published in the peer reviewed journal: The Proceedings of the National Academy of Sciences (PNAS).
https://www.pnas.org/doi/full/10.1073/pnas.2317026121
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
