BREAKING COVID-19 News! Study Finds That Omicron Variants Have A Unique Ability To Induce Cellular Senescence!
Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 16, 2023 1 year, 3 months, 4 weeks, 2 days, 7 hours, 35 minutes ago
COVID-19 News: As the COVID-19 pandemic continues its relentless march across the globe, the scientific community remains vigilant in the face of new challenges posed by emerging SARS-CoV-2 variants. A recent collaboration between Jena University Hospital in Germany and the University of California San Francisco in the USA has brought to light a groundbreaking discovery - the omicron variant's unique ability to induce premature cellular senescence. This revelation not only deepens our understanding of the virus but also raises critical questions about its implications for disease severity, long-term complications, and the ongoing efforts to curb the pandemic.
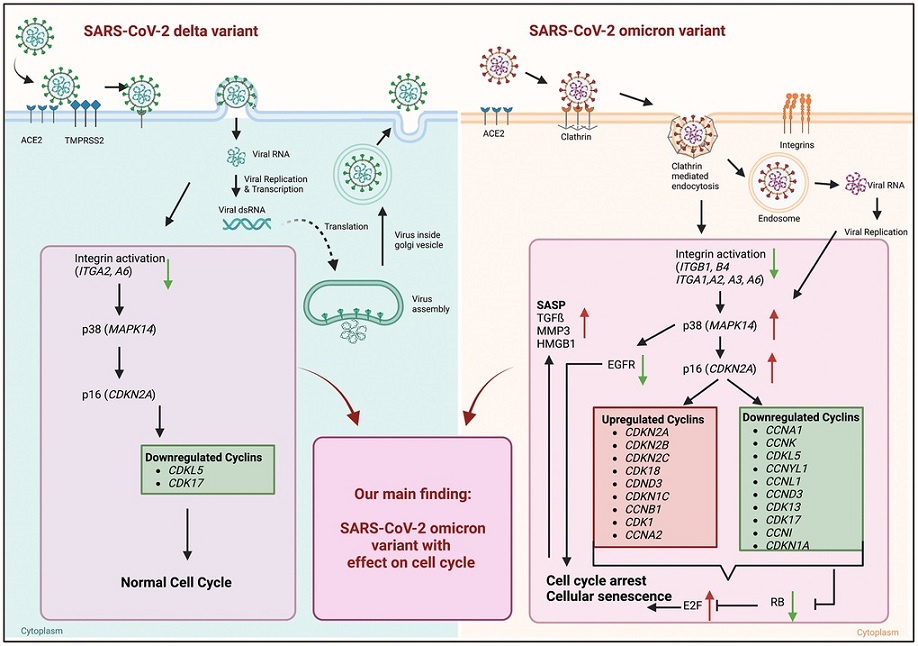 Graphical abstract of known differences of SARS-CoV-2 delta and omicron variant entry and findings of our study obtained from mRNA sequence data of 24 h post infection. Schematic overview of entry mechanisms of SARS-CoV-2 delta and omicron variants [5]. Delta variant (right panel) uses cell surface entry, by ACE2 and the protease TMPRSS2. Own data indicate a downregulation of the integrin activation without affecting p38 and p16 expression resulting in normal cell cycle. Omicron variant (right panel) prefers to use the clathrin-mediated endocytosis (CME) and cathepsin L as protease. Our results suggest the expression and activation of integrins (ITGB1, ITGB4, ITGA1, ITGA2, ITGA3, ITGA6) resulting an increase in p38 and p16. That increase in central kinases affects several cyclins, which in turn downregulates the retinoblastoma, increases the E2F transcription factors and results in cell cycle arrest and cellular senescence. Additionally, these changes lead to an increase in senescence-associated secretory phenotype (SASP). Thus, our findings indicate an influence of the altered cell entry mechanism of the omicron variant on the cell cycle.
The Ever-Evolving Landscape of SARS-CoV-2 Variants
Graphical abstract of known differences of SARS-CoV-2 delta and omicron variant entry and findings of our study obtained from mRNA sequence data of 24 h post infection. Schematic overview of entry mechanisms of SARS-CoV-2 delta and omicron variants [5]. Delta variant (right panel) uses cell surface entry, by ACE2 and the protease TMPRSS2. Own data indicate a downregulation of the integrin activation without affecting p38 and p16 expression resulting in normal cell cycle. Omicron variant (right panel) prefers to use the clathrin-mediated endocytosis (CME) and cathepsin L as protease. Our results suggest the expression and activation of integrins (ITGB1, ITGB4, ITGA1, ITGA2, ITGA3, ITGA6) resulting an increase in p38 and p16. That increase in central kinases affects several cyclins, which in turn downregulates the retinoblastoma, increases the E2F transcription factors and results in cell cycle arrest and cellular senescence. Additionally, these changes lead to an increase in senescence-associated secretory phenotype (SASP). Thus, our findings indicate an influence of the altered cell entry mechanism of the omicron variant on the cell cycle.
The Ever-Evolving Landscape of SARS-CoV-2 Variants
The SARS-CoV-2 virus, responsible for the global COVID-19 pandemic, has undergone numerous mutations, leading to the emergence of variants of concern (VOC). Notable variants include alpha (B.1.1.7), delta (B.1.617.2), and the more recent omicron (B.1.1.529). These variants often exhibit changes in the spike protein, a key player in viral entry into host cells. The omicron variant, in particular, has drawn attention due to its distinctive endosomal clathrin-mediated entry mechanism, setting it apart from its predecessors.
Understanding the Influence of Altered Spike Formations on Cellular Senescence
The research covered in this
COVID-19 News report, aimed to unravel the impact of altered spike formations on cellular senescence - a state characterized by irreversible cell cycle arrest, altered metabolism, and the release of pro-inflammatory signals known as the senescence-associated secretory phenotype (SASP).
Methods and Results of the Study
To explore the connection between spike protein variations and cellular senescence, the researchers conducted in vitro infections using SARS-CoV-2 delta and omicron variants. Human primary small alveolar epithelial cells and human ex vivo lung slic
es served as the experimental models. Confirming the presence of cellular senescence in the lungs of COVID-19 patients, the study utilized mRNA sequencing to identify global gene expression patterns in infected human primary alveolar epithelial cells.
The results were striking. Solely the omicron variants of SARS-CoV-2 demonstrated a significant impact on the expression of cell cycle genes, prominently marked by increased p21 expression in both human primary lung cells and ex vivo lungs. Moreover, an upregulated SASP was detected, indicative of a distinct cellular response. Transcriptomic data revealed heightened gene expression of p16 and p38 in omicron-infected lung cells, signaling substantial alterations in cell cycle, inflammation, and integrin-associated pathways, collectively pointing towards premature cellular senescence.
Connecting the Dots: Integrating Entry Mechanisms and Senescence
The unique entry mechanism of the omicron variant, reliant on endosomal clathrin-mediated entry, distinguishes it from other variants. This mechanism involves the activation of integrins, a class of cell adhesion molecules
. Notably, recent studies have illuminated the connection between altered molecular regulation in endocytic pathways, integrin activation, and cellular senescence.
Persistent activation of integrins, resulting from downregulated clathrin-mediated endocytosis, has been linked to increased senescence-related gene expression.
Previous research has already demonstrated the ability of SARS-CoV-2 to induce senescence both in vitro and in vivo, with COVID-19 patients exhibiting signs of cellular senescence. However, the unique contribution of the omicron variant to this senescent phenotype has been a key focus of the latest study.
The Paradox of Severity: Clinical Outcomes and Omicron
The study's findings raise intriguing questions about the relationship between the observed senescent phenotype induced by the omicron variant and clinical outcomes. Despite displaying a robust senescent response, omicron infections have presented with milder respiratory symptoms and lower disease severity compared to the delta variant.
This paradoxical observation challenges conventional wisdom and underscores the complexity of the interplay between viral variants, host responses, and clinical manifestations. While the core symptoms of COVID-19 remain consistent, the distinct characteristics of the omicron variant have led to a lower risk of severe disease and hospitalization. Several factors, including potential changes in the virus's spike protein and a higher level of pre-existing immunity in the population, are attributed to the observed differences in disease severity.
Long-Term Implications: Senescence and Post-COVID Complications
The study also delves into the long-term implications of SARS-CoV-2 infections, particularly the relationship between the severity of infection and the occurrence of Long-COVID. While omicron has been associated with a lower risk of severe disease, the research emphasizes the need for continued investigation into the differences between omicron and delta infections concerning post-COVID complications, including lung fibrosis.
Comparisons of long-term sequelae resulting from infections with different SARS-CoV-2 variants have revealed varying degrees of lung damage and fibrosis. Some studies suggest that COVID-19 may lead to progressive lung damage, with omicron infections showing a distinct pattern of milder respiratory symptoms and less involvement of the lower respiratory tract. However, the literature remains inconclusive regarding the differential impact of omicron and delta variants on the pathogenesis of pulmonary fibrosis.
Connecting Inflammaging to Senescence
The study's findings align with the concept of inflammaging - a proposed phenomenon where heightened levels of local and systemic pro-inflammatory cytokines associated with aging contribute to the development of the cytokine storm observed during severe COVID-19. The observed combination of cell cycle arrest and increasing levels of SASP factors in response to SARS-CoV-2 infection supports the notion that the virus-induced senescence may contribute to the pro-inflammatory environment observed in severe cases.
The study goes on to emphasize the potential link between the induction of cellular senescence in lung tissue and the reported cytokine storm, a severe inflammatory response often associated with respiratory distress in COVID-19 patients. The distinctive senescent phenotype induced by the omicron variant may provide clues to the mechanisms underlying the reported cytokine storm and contribute to our understanding of the development of long-term COVID-19 complications.
Conclusion
In conclusion, the collaborative research effort has unveiled a significant breakthrough in our understanding of the omicron variant's unique pathogenic mechanism. The induction of premature cellular senescence, as evidenced by distinct gene expression patterns and senescence-associated phenotypes, sets the omicron variant apart from its predecessors.
The paradoxical observation of milder clinical outcomes despite the induction of a robust senescent response raises intriguing questions about the complex relationship between viral variants, host responses, and disease severity. As the global scientific community continues to grapple with the challenges posed by SARS-CoV-2 variants, this study provides a critical piece of the puzzle, urging further investigation into the intricate mechanisms at play.
As we navigate the evolving landscape of the COVID-19 pandemic, understanding the implications of cellular senescence induced by the omicron variant becomes crucial. This knowledge not only informs our efforts in developing targeted therapeutic strategies but also underscores the importance of ongoing research in unraveling the mysteries of SARS-CoV-2 and its impact on human health. The journey towards defeating the pandemic requires collaborative, multidisciplinary efforts, and each discovery brings us closer to a comprehensive understanding of the virus and its variants.
The study findings were published in the peer reviewed journal: Aging.
https://www.aging-us.com/article/205297/text
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
