BREAKING! Emerging SARS-CoV-2 Variants Are Learning To Suppress Immune Responses Against Itself And Original Strains Helps Via Negative Immune Imprinting!
Source: SARS-CoV-2 Immunity Research Jun 16, 2022 3 years, 6 months, 4 days, 8 hours, 56 minutes ago
SARS-CoV-2 Immunity Research: New disturbing study findings by researchers from the United Kingdom shows that emerging SARS-CoV-2 variants are learning to suppress immune responses against itself and the original strains and earlier variants that causes initial waves in this ongoing pandemic helps these new emerging variants via negative immune imprinting!
The study findings indicate that individuals who contracted the COVID-19 disease during the first wave of the pandemic get no boost to their immune response if they subsequently catch Omicron.
The
SARS-CoV-2 Immunity Research findings also shows that although three doses of a COVID jab may help to protect people from severe outcomes if they catch the initial Omicron variants, previous infections can affect their immune response.
In simple terms as explained by the co-author of the study, Professor Dr Rosemary Boyton from Imperial College London-UK, “If you were infected during the first wave, then you can’t boost your immune response if you have an Omicron infection.”
The study team also found an Omicron infection offered little extra protection against catching the variant again.
Professor Boyton added, “When Omicron started flying around the country, people kept saying that’s OK, that will improve people’s immunity. Out study findings show that it’s not a good booster of immunity.”
T cells are supposed to kill infected cells however the SARS-CoV-2 destroys the functional aspects of how T cells work and recognize infected cells hence after a COVID-10 infections, not only are these T-Cells dysfunctional and not able to protect one form future infections, in some cases they may trigger auto-immune conditions and end up destroying healthy cells as what we are seeing in Long COVID.
The Omicron variant (B.1.1.529) carries multiple spike mutations with high transmissibility and partial neutralizing antibody (nAb) escape. Vaccinated individuals show protection from severe disease, often attributed to primed cellular immunity.
The study team investigated T and B cell immunity against B.1.1.529 in triple mRNA vaccinated healthcare workers (HCW) with different SARS-CoV-2 infection histories.
The study findings showed that B and T cell immunity against previous variants of concern was enhanced in triple vaccinated individuals, but magnitude of T and B cell responses against B.1.1.529 spike protein was reduced.
Alarmingly, immune imprinting by infection with the earlier B.1.1.7 (Alpha) variant resulted in less durable binding antibody against B.1.1.529.
Previously infection-naïve HCW who became infected during the B.1.1.529 wave showed enhanced immunity against earlier variants, but reduced nAb potency and T cell responses against B.1.1.529 itself.
The study findings also showed that previous Wuhan Hu-1 infection abrogated T cell recognition and any enhanced cross-reactive neutralizing immunity on infection with B.1.1.529.
The study findings were published in the peer reviewed journal: Science
.org/doi/10.1126/science.abq1841">https://www.science.org/doi/10.1126/science.abq1841
The new study findings may help to explain why reinfections with Omicron over a short time period have been so frequent.
The study findings are also critical for vaccine development strategies.
The study team followed the vaccination and infection experiences of 731 triple vaccinated healthcare workers in the UK from March 2020 to January 2022. The study team then used blood samples collected from participants in the weeks after their third dose of vaccine to explore their antibody and T-cell responses towards the Omicron variant, BA.1.
The research participants were screened considerably in terms of their COVID history, including whether they had had a previous COVID infection and, if so, the variant involved.
The study findings alarmingly indicated that irrespective of the participants’ previous infection history, a few weeks after their third COVID jab… their levels of T-cells against Omicron proteins were poor, while levels of antibodies against Omicron proteins were lower than against other variants.
The study team told Thailand
Medical News, “Our T cell analysis, which depended on processing of immunodominant epitopes from whole antigen, revealed a more profound deficit than others. Studies in which T cell responses of vaccinees against spike peptide megapools are screened show that, while there may be a 20% drop in response due to lost epitopes across the entire sequence, most of the response is maintained, albeit with a significant minority showing a completely ablated CD8 response to Omicron peptide pools!”
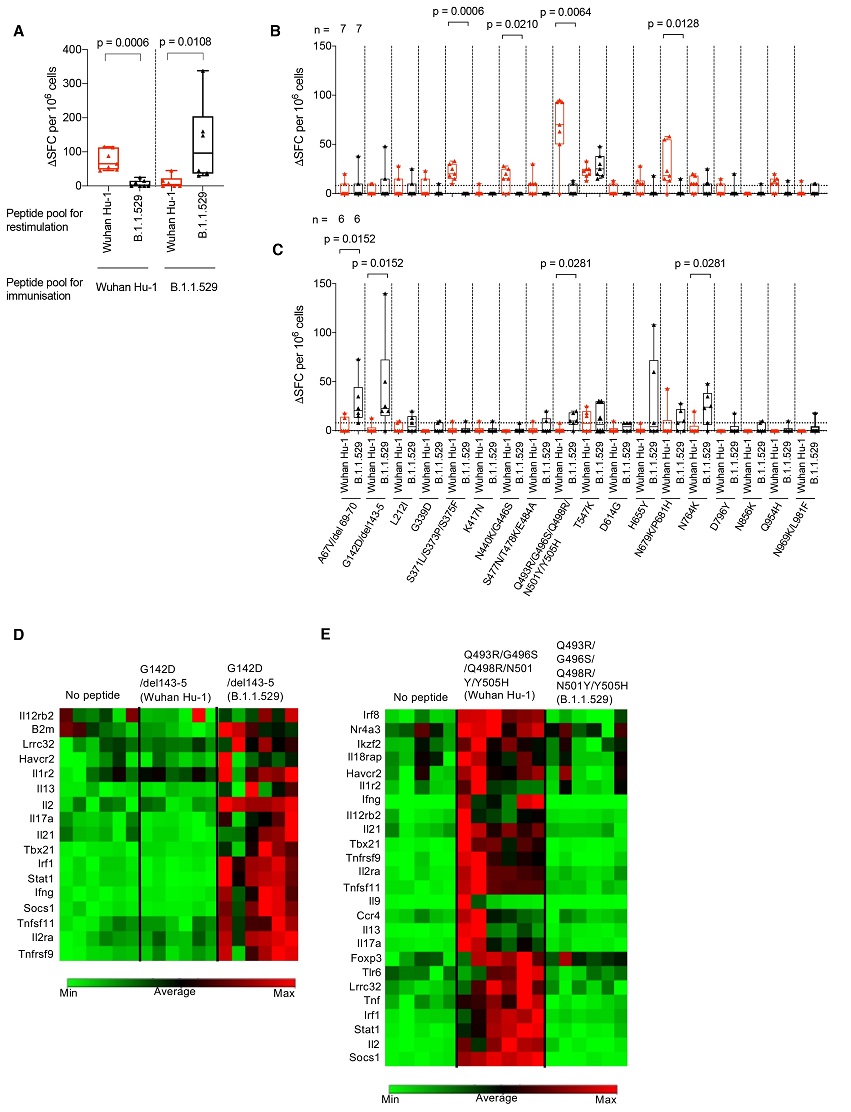 B.1.1.529 (Omicron) spike mutations alter T cell recognition.
(A) HLAII transgenic mice carrying DRB1*0401 in the context of a homozygous knockout for murine H2-Aβ (7-8w) were immunized with either a B.1.1.529 (Omicron, n = 7) VOC pool of 18 peptides encompassing the Omicron sequence mutations or the ancestral Wuhan Hu-1 pool of peptides (n = 6) with the equivalent unmutated sequences. At d10 DLN cells were prepared from immunized mice and stimulated with either Wuhan Hu-1 (red) or B.1.1.529 (Omicron, black) peptide pools and T cell responses measured by IFNγ ELISpot. (B) IFNγ T cell responses were mapped against individual Wuhan Hu-1 (red) or B.1.1.529 (Omicron, black) peptides using DLN taken from Wuhan Hu-1 peptide pool (n = 7) or (C) B.1.1.529 (Omicron) peptide pool (n = 6) immunized mice. (D and E) Heatmaps showing relative gene expression of T cell activation markers in DLN cells taken from B.1.1.529 (Omicron) G142D/del143-5 peptide primed (D) (n = 6) or Wuhan Hu-1 Q493R/G496S/Q498R/N501Y/Y505H peptide primed (E) (n = 6) HLA-DRB1*04:01 transgenic mice. DLN cells were stimulated for 24h in vitro with 10 μg/ml Wuhan Hu-1 or B.1.1.529 (Omicron) variant peptide before RNA extraction. Genes shown in the heatmap are significantly up-regulated (p < 0.05) in Wuhan Hu-1 or B.1.1.529 (Omicron) variant peptide stimulated cells compared to no peptide control. Statistical tests were performed using Prism 9.0 or the Qiagen GeneGlobe data analysis tool for gene expression data. [(A) to (C)] Wilcoxon matched-pairs signed rank test, [(D) and (E)] Student’s t test. DLN, draining lymph node; SFC, spot forming cells; h, hours; VOC, variant of concern.
B.1.1.529 (Omicron) spike mutations alter T cell recognition.
(A) HLAII transgenic mice carrying DRB1*0401 in the context of a homozygous knockout for murine H2-Aβ (7-8w) were immunized with either a B.1.1.529 (Omicron, n = 7) VOC pool of 18 peptides encompassing the Omicron sequence mutations or the ancestral Wuhan Hu-1 pool of peptides (n = 6) with the equivalent unmutated sequences. At d10 DLN cells were prepared from immunized mice and stimulated with either Wuhan Hu-1 (red) or B.1.1.529 (Omicron, black) peptide pools and T cell responses measured by IFNγ ELISpot. (B) IFNγ T cell responses were mapped against individual Wuhan Hu-1 (red) or B.1.1.529 (Omicron, black) peptides using DLN taken from Wuhan Hu-1 peptide pool (n = 7) or (C) B.1.1.529 (Omicron) peptide pool (n = 6) immunized mice. (D and E) Heatmaps showing relative gene expression of T cell activation markers in DLN cells taken from B.1.1.529 (Omicron) G142D/del143-5 peptide primed (D) (n = 6) or Wuhan Hu-1 Q493R/G496S/Q498R/N501Y/Y505H peptide primed (E) (n = 6) HLA-DRB1*04:01 transgenic mice. DLN cells were stimulated for 24h in vitro with 10 μg/ml Wuhan Hu-1 or B.1.1.529 (Omicron) variant peptide before RNA extraction. Genes shown in the heatmap are significantly up-regulated (p < 0.05) in Wuhan Hu-1 or B.1.1.529 (Omicron) variant peptide stimulated cells compared to no peptide control. Statistical tests were performed using Prism 9.0 or the Qiagen GeneGlobe data analysis tool for gene expression data. [(A) to (C)] Wilcoxon matched-pairs signed rank test, [(D) and (E)] Student’s t test. DLN, draining lymph node; SFC, spot forming cells; h, hours; VOC, variant of concern.
The study findings showed that previous infections also mattered.
The study team reported infection with Omicron increased protection against future infection with other variants. However, it only offered a limited boost to protection against another Omicron infection…a response that was actually weakened among those who had also previously had the original strain of the virus.
The study team said the results held for both antibody and T-cell responses, and suggested those who caught a SARS-CoV-2 infection in the first wave of the pandemic did not gain a boost to their immune response should they subsequently catch Omicron.
The study team said the finding was a surprise as it was typically assumed that a prior infection, even of a different variant, would act to boost an individual’s immune response.
Co-researcher, Professor Danny Altmann from the Hammersmith Hospital Campus of Imperial College London warned that while it had previously been thought SARS-CoV-2 variants such as Omicron had developed mutations in their spike protein that helped them to evade immune responses, the situation was more complex.
He added, “It’s actually worse than that, because the adaptations that the spike protein has now are actually inducing a kind of regulation or shutdown of immune response!”
Although the study looked at responses to the BA.1, similar findings were likely for other subvariants of Omicron.
In fact, with the more divergent BA.4 and BA.5 variants and their subvariants, the findings might be far worse.
The study team added that with individuals in the UK having had very different histories of SARS-CoV-2 infections and vaccinations, the study was important as it suggested this “immune imprinting” would shape subsequent immunity against the next variant.
Professor Altmann said that while the continued low levels of hospitalization and deaths from COVID in the UK, despite high levels of infection, suggested COVID jabs continued to offer protection against death and severe disease, the findings could be important for the development of new vaccines.
However, he added the study findings raised other concerns and commented, “We’re not getting herd immunity, we’re not building up protective immunity to Omicron. So, we face not coming out the other end of infections and re-infections and breakthrough infections.”
The study team concluded, “In summary, these studies have shown that the high global prevalence of B.1.1.529 (Omicron) infections and reinfections likely reflects considerable subversion of immune recognition at both the B, T cell, antibody binding and nAb level, although with considerable differential modulation through immune imprinting. Some imprinted combinations, such as infection during the Wuhan Hu-1 and Omicron waves, confer particularly impaired responses!”
For the latest
SARS-CoV-2 Immunity Research, keep on logging to Thailand
Medical News.

