BREAKING! French Scientists From Aix Marseille University Discover That Vimentin Is A Critical ACE2 Co-Receptor For SARS-CoV-2 In Epithelial Cells!
Source: Medical News - Vimentin Co-Receptor For SARS-CoV-2 Nov 02, 2022 2 years, 5 months, 2 weeks, 4 days, 7 hours, 40 minutes ago
A new study by researchers from Aix Marseille University -France has discovered that Vimentin, a host structural protein is also an important ACE2 Co-Receptor for SARS-CoV-2 in epithelial cells!
Thailand
Medical News had also reported on a previous American study that also found that Vimentin plays a role in SARS-CoV-2 infections.
https://www.thailandmedical.news/news/breaking-boston-university-discovers-that-vascular-protein-vimentin-assists-sars-cov-2-access-into-cells,-contributing-to-vascular-complications
The host protein Vimentin is a type III intermediate filament protein, widely expressed in mesenchymal cells. Mainly located in the cytoplasm, vimentin can also appear at extracellular locations, where it may interact with bacterial or viral pathogens. I
The French study team initially aimed at investigating the implication of vimentin in SARS-CoV-2 viral entry and the consequences on viral replication and cellular response.
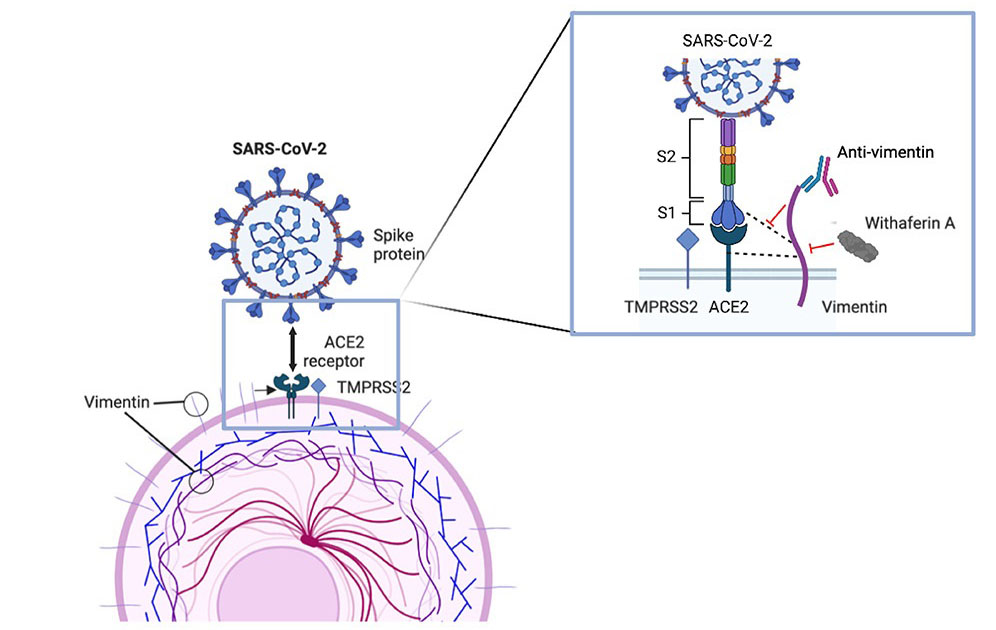
The study findings showed that upon infection, vimentin was upregulated at the cell surface, where it interacts with ACE2 for SARS-CoV-2 entry.
The study findings also demonstrated a direct interaction between SARS-CoV-2 spike protein, ACE2 and vimentin in epithelial cells.
Importantly inhibition of cell surface vimentin availability resulted in reduced viral entry and cytopathogenic effects.
The study findings also showed that the expression of inflammatory cytokines and chemokines was modulated by vimentin - SARS-CoV-2 interaction.
The study findings suggest that cell surface vimentin acts as a co-receptor for SARS-CoV-2.
The study findings were published in the peer reviewed journal: iScience.
https://www.cell.com/iscience/fulltext/S2589-0042(22)01735-7
Vimentin is a structural protein that in humans is encoded by the VIM gene. Its name comes from the Latin vimentum which refers to an array of flexible rods. It is a type III intermediate filament (IF) protein that is expressed in mesenchymal cells. IF proteins are found in all animal cells as well as bacteria.
Intermediate filaments, along with tubulin-based microtubules and actin-based microfilaments, comprises the cytoskeleton. All IF proteins are expressed in a highly developmentally-regulated fashion; vimentin is the major cytoskeletal component of mesenchymal cells. Because of this, vimentin is often used as a marker of mesenchymally-derived cells or cells undergoing an epithelial-to-mesenchymal transition (EMT) during both normal development and metastatic progression.
Vimentin plays a significant role in supporting and anchoring the position of the organelles in the cytosol. Vimentin is attached to the nucleus, endoplasmic reticulum, and mitochondria, either laterally or terminally.
The dynamic nature of vimentin is important when offering flexibility to the cell. Sci
entists found that vimentin provided cells with a resilience absent from the microtubule or actin filament networks, when under mechanical stress in vivo. Therefore, in general, it is accepted that vimentin is the cytoskeletal component responsible for maintaining cell integrity. (It was found that cells without vimentin are extremely delicate when disturbed with a micropuncture).
Transgenic mice that lack vimentin appeared normal and did not show functional differences. It is possible that the microtubule network may have compensated for the absence of the intermediate network. This result supports an intimate interaction between microtubules and vimentin. Moreover, when microtubule depolymerizers were present, vimentin reorganization occurred, once again implying a relationship between the two systems. On the other hand, wounded mice that lack the vimentin gene heal slower than their wild type counterparts.
Vimentin has also been found to control the transport of low-density lipoprotein, LDL, -derived cholesterol from a lysosome to the site of esterification.With the blocking of transport of LDL-derived cholesterol inside the cell, cells were found to store a much lower percentage of the lipoprotein than normal cells with vimentin. This dependence seems to be the first process of a biochemical function in any cell that depends on a cellular intermediate filament network. This type of dependence has ramifications on the adrenal cells, which rely on cholesteryl esters derived from LDL.
Vimentin has been used as a sarcoma tumor marker to identify mesenchyme.
Methylation of the vimentin gene has been established as a biomarker of colon cancer and this is being utilized in the development of fecal tests for colon cancer. Statistically significant levels of vimentin gene methylation have also been observed in certain upper gastrointestinal pathologies such as Barrett's esophagus, esophageal adenocarcinoma, and intestinal type gastric cancer. High levels of DNA methylation in the promoter region have also been associated with markedly decreased survival in hormone positive breast cancers. Downregulation of vimentin was identified in cystic variant of papillary thyroid carcinoma using a proteomic approach.
The key findings from the French study are:
-SARS-CoV-2 upregulates vimentin surface expression
-SARS-CoV-2 spike interacts with Vimentin and ACE2 at the cell surface
-Cell surface vimentin favors SARS-CoV-2 infection
-Vimentin inhibition protects viral-induced cytotoxicity and modulates host response
It has already been established that both SARS-CoV and SARS-CoV-2 are able to bind to angiotensin-converting enzyme 2 (ACE2), which catalyzes the cleavage of angiotensin II into the vasodilator angiotensin 1-7. ACE2 is a membrane bond metallopeptidase which is the key receptor for the entry of SARS55 CoV-2 into host cells. The S1 subunit of the spike (S) protein of SARS-CoV-2 binds to ACE2 which initiates the priming of the SARS-CoV-2-S protein by Transmembrane serine protease 2 (TMPRSS2), allowing virus-cell fusion and cell entry. However, ACE2 is poorly expressed throughout the respiratory tract, suggesting that other cofactors may compensate and facilitate the interactions between S protein and ACE2. For example, Neuropilin-1 (NRP1) has beenshown to enhance the interaction between ACE2 and S protein. CD209L/L-SIGN, 61 CD209/DC-SIGN, and heparan sulfate may also facilitate SARS-CoV-2 entry into lung cells. Finally, vimentin was shown to interact with SARS-CoV S protein at the cell surface.
https://pubmed.ncbi.nlm.nih.gov/26801988/
Vimentin is a type III intermediate filament cytoskeletal protein expressed in non-muscle cells, including fibroblasts, endothelial cells, macrophages, melanocytes, Schwann cells and lymphocytes. The basic structure of vimentin consists of a central α-helical rod domain flanked by unstructured head and tail domains. Vimentin forms a vast intracellular, dynamic and flexible network surrounding the nucleus and spanning towards the cell periphery which allows maintenance of the cell’s organelles and plays an important role in several cell events
Viruses have the ability to infect target cells following interaction with specific cell surface receptors. Importantly, assistance of non-specific co receptors may increase receptor affinity, favor infection efficiency and contribute to the tropism.
Based on its high homology with SARS-Co-V, it was rapidly demonstrated that SARS-CoV-2 is endocytosed after engagement of ACE2 by its spike protein. However, many studies have revealed that depending on the cell type, SARS-CoV-2 entry may involve other cell surface molecules or attachment factors. For example, heparan sulfate or sialic acids potentially favor interaction of the spike RBD with ACE2. In addition, it was shown that neuropilins, and particularly neuropilin 1 (NRP1), which is highly expressed on the respiratory epithelium serves as a host factor for infection. Finally, alternative receptors, including CD147 and GRP78 have been proposed to define novel tropism.
The study team showed that similar to SARS-CoV, cell-surface vimentin acts as a co-receptor or an attachment factor for SARS-CoV-2 infection.
The study findings are strengthened by conclusions from recent reports which showed that vimentin was involved during infection with SARS-CoV-2.
https://pubmed.ncbi.nlm.nih.gov/35078919/
https://pubmed.ncbi.nlm.nih.gov/34866333/
https://www.nature.com/articles/s41598-022-11248-y
Significantly, the study findings showed that efficient infection required both cell-surface vimentin and ACE2. Indeed, cells expressing high levels of ACE2 but no vimentin (Caco-2 cells) were infected with SARS-CoV-2 but not as efficiently as cells expressing high levels of both ACE2 and vimentin (Vero E6 cells).
Furthermore, ACE2 expression was required since cells expressing no/low levels of ACE2 but high cell-surface vimentin (A549 cells) were barely infected by SARS-CoV-2.
The study findings in agreement with another past study that showed co-expression of vimentin with ACE2 in HEK293 cells resulted in increased infection while overexpression of vimentin alone had only a modest effect on cell infection.
https://pubmed.ncbi.nlm.nih.gov/35078919/
Also, by combining colocalization studies by confocal microscopy and coimmunoprecipitation experiments, the study team also provided evidence that ACE2 and vimentin interacted directly and that this interaction only occurred in the presence of SARS-CoV-2, suggesting that vimentin, ACE2 and SARS-CoV-2 spike protein form a trimolecular complex, as previously hypothesized with SARS-CoV.
https://pubmed.ncbi.nlm.nih.gov/26801988/
The study findings also showed that that vimentin inhibition was accompanied by a significant reduction of SARS-CoV-2-induced cytopathic effects, and by an increase of cell viability. Thus, the role of vimentin at later stages of SARS-CoV-2 infection, including viral replication, assembly and egress needs to be further explored.
The study findings also showed that treatment of cells with anti-vimentin antibodies reduced the expression of chemokines and cytokines, including CCL5, CXCL10 and IL-6. IL-6 is a pleiotropic cytokine with multiple activities on inflammation and immunity. It has been shown that SARS-CoV-2 spike protein induces IL311 6 expression in an angiotensin II type 1 (AT1)-dependent signaling resulting from ACE2 downregulation.
It should be noted that IL-6 is one of the major headliners of the cytokine storm which accelerates the severity of the infection by SARS-CoV-2. In addition, vimentin has been shown necessary for the assembly and activation of the NLRP3 inflammasome, which is involved in the production of pro-inflammatory cytokines.
The study findings suggest that beside reducing viral entry, targeting vimentin may also reduce the risk of cytokine storm syndrome and thus prevent evolution towards severe COVID-19.
Overall, the study findings showed that vimentin inhibition reduced viral uptake, protect against virus-mediated cell cytotoxicity, promote cell survival, and reduced pro-inflammatory response. I
Importantly, similar data were obtained when cells were infected with the Omicron variant.
Past studies had also shown that anti-vimentin antibodies also reduced the uptake of pseudoviruses expressing spike proteins from the variants UK B.1.1.7 and Brazil P.1.
https://pubmed.ncbi.nlm.nih.gov/34866333/
The study team says that as such, drugs targeting vimentin, including ALD-R491, a novel oral, fast-acting, and non-cytotoxic molecule appear as promising and lasting candidates for the treatment of SARS-CoV-2 infections and COVID-19.
https://pubmed.ncbi.nlm.nih.gov/34634931/
Thailand
Medical News would also like to add that Artemisinins extracted from the herb wormwood also target vimentin.
https://www.jbc.org/article/S0021-9258(17)48908-3/pdf
For the latest
SARS-CoV-2 Research, keep on logging to Thailand
Medical News.
Read Also:
COVID-19 Herbs
https://www.thailandmedical.news/articles/covid-19-herbs
