BREAKING! German Study Finds That SARS-CoV-2 Is Using Endosomes For Viral Entry!
Nikhil Prasad Fact checked by:Thailand Medical News Team Jun 03, 2024 1 year, 6 months, 3 weeks, 6 days, 23 hours, 41 minutes ago
COVID-19 News: In a significant scientific breakthrough, a German research team has identified a novel pathway through which the SARS-CoV-2 virus enters host cells. This discovery, spearheaded by Dr Richard Brown and Dr Daniel Todt's Computational Virology group at Ruhr University Bochum, in collaboration with the Paul-Ehrlich-Institut, has profound implications for our understanding of COVID-19 transmission and progression. The study covered in this
COVID-19 News report, reveals that the TMPRSS2 protein facilitates viral entry into cells via endosomes, challenging previous assumptions about the virus’s entry mechanisms.
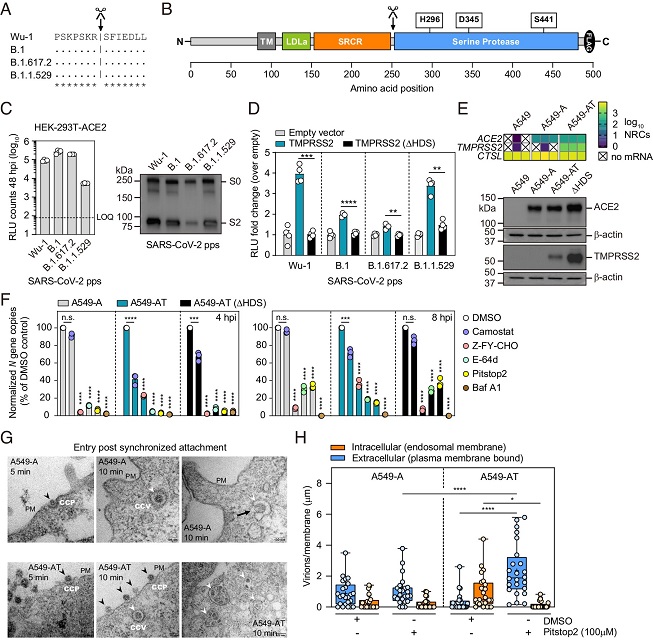 TMPRSS2 enhances SARS-CoV-2 internalization into endosomes. (A) Conservation of S2’ site between variants. (B) Cartoon of human TMPRSS2 protein domains. Location of catalytic triad residues and autocleavage site (scissors) are highlighted above. (C) SARS-CoV-2 pp cell entry of the indicated S variants. Left panel: RLU counts at in HEK-293T-ACE2 cells at 48 hpi. RLU: Relative light units. LOQ: limit of quantification. (N = 4 mean ± SEM). Right panel: Western blot quantification of S protein expression in SARS-CoV-2 containing supernatants. (D) Uptake of the indicated SARS-CoV-2 pps in the presence of TMPRSS2 or TMPRSS2 (ΔHDS). (N = 4 mean ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001). (E) Human ACE2 and TMPRSS2 mRNA (Top, N = 3) and protein expression (Bottom) in the indicated cell lines. (F) Authentic B.1 vRNA levels in the indicated cell lines at 4 hpi (Left) and 8 hpi (Right) in the presence of the indicated inhibitors, normalized to vRNA levels in DMSO control treated cells. MOI = 0.01. N = 3 (mean ± SEM). (G) EM visualization of authentic B.1 virus entry into A549-A and A549-AT cells. Virus uptake by endocytosis is observed at 5 and 10 min in both cell lines, with B.1 binding to CCPs (Left and Middle panels; black arrowheads). In both cell lines at 10 min, B.1 is observed in CCVs (Middle and Right panels; white arrowheads) and endocytic compartments (Right panels; white arrowheads). In A549-A cells at 10 min (Top Right), the black arrow highlights a virus–host membrane fusion event. In A549-AT cells at 10 min (Bottom Right) clusters of virions accumulate in endosomal compartments (white arrowheads). PM: plasma membrane; CCV: clathrin-coated vesicle; CCP: clathrin-coated pit. (H) Quantification of extracellular and internalized virions. Normalized virion counts per µm/membrane for extracellular plasma membrane–associated virions (blue) or internalized endosomal virions (orange). Infections were performed as in (G), in the presence of either DMSO or Pitstop 2 (100 µM), and image quantification was performed at 10 min post temperature shift. MOI = 500.
TMPRSS2: A Key Player in Viral Entry
The team’s research centers around the role
TMPRSS2 enhances SARS-CoV-2 internalization into endosomes. (A) Conservation of S2’ site between variants. (B) Cartoon of human TMPRSS2 protein domains. Location of catalytic triad residues and autocleavage site (scissors) are highlighted above. (C) SARS-CoV-2 pp cell entry of the indicated S variants. Left panel: RLU counts at in HEK-293T-ACE2 cells at 48 hpi. RLU: Relative light units. LOQ: limit of quantification. (N = 4 mean ± SEM). Right panel: Western blot quantification of S protein expression in SARS-CoV-2 containing supernatants. (D) Uptake of the indicated SARS-CoV-2 pps in the presence of TMPRSS2 or TMPRSS2 (ΔHDS). (N = 4 mean ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001). (E) Human ACE2 and TMPRSS2 mRNA (Top, N = 3) and protein expression (Bottom) in the indicated cell lines. (F) Authentic B.1 vRNA levels in the indicated cell lines at 4 hpi (Left) and 8 hpi (Right) in the presence of the indicated inhibitors, normalized to vRNA levels in DMSO control treated cells. MOI = 0.01. N = 3 (mean ± SEM). (G) EM visualization of authentic B.1 virus entry into A549-A and A549-AT cells. Virus uptake by endocytosis is observed at 5 and 10 min in both cell lines, with B.1 binding to CCPs (Left and Middle panels; black arrowheads). In both cell lines at 10 min, B.1 is observed in CCVs (Middle and Right panels; white arrowheads) and endocytic compartments (Right panels; white arrowheads). In A549-A cells at 10 min (Top Right), the black arrow highlights a virus–host membrane fusion event. In A549-AT cells at 10 min (Bottom Right) clusters of virions accumulate in endosomal compartments (white arrowheads). PM: plasma membrane; CCV: clathrin-coated vesicle; CCP: clathrin-coated pit. (H) Quantification of extracellular and internalized virions. Normalized virion counts per µm/membrane for extracellular plasma membrane–associated virions (blue) or internalized endosomal virions (orange). Infections were performed as in (G), in the presence of either DMSO or Pitstop 2 (100 µM), and image quantification was performed at 10 min post temperature shift. MOI = 500.
TMPRSS2: A Key Player in Viral Entry
The team’s research centers around the role of the TMPRSS2 protein in SARS-CoV-2 entry. TMPRSS2 acts as a helper protein, enhancing the virus's ability to enter host cells by interacting with
the ACE2 receptor. This interaction significantly alters the host cells' immune response and accelerates the virus's evolution. Notably, the TMPRSS2-mediated pathway is effective not only in humans but also in various animals, including wild, domestic, and farm species. This suggests a broader ecological impact and a potential influence on zoonotic transmission.
Unexpected Role of Endosomes
Traditionally, it was believed that TMPRSS2-assisted viral entry bypassed endosomes, cellular vesicles involved in transport and sorting within cells. However, using electron microscopy, the researchers discovered that TMPRSS2 actually facilitates the uptake of the virus into endosomes. "Contrary to previous assumptions, TMPRSS2-mediated cell entry is associated with increased virus uptake into specific cellular vesicles: endosomes," explained Dr. Brown. This unexpected finding necessitates further detailed studies to fully understand the implications of endosome involvement in viral entry.
Implications for Virus Evolution and Host Immune Response
The presence of TMPRSS2 not only enhances viral entry but also impacts what happens inside the cell post-entry. The research demonstrated that efficient TMPRSS2-mediated entry leads to increased viral genome replication and higher virus production. Consequently, infected cells exhibit a stronger immune response, resulting in quicker cell death. This heightened immune response influences the virus's evolution, favoring mutations that can better evade the immune system.
The researchers also observed that the TMPRSS2-enhanced entry mechanism applies to various SARS-CoV-2 variants, including the more recent Omicron variant. Even though the Omicron variant does not require TMPRSS2 for cell entry, the presence of TMPRSS2 still significantly increases viral uptake. This discovery has profound implications for understanding the evolution and spread of different SARS-CoV-2 variants.
Broader Mammalian Impact
The study extends beyond human hosts, demonstrating that the TMPRSS2 protein can enhance SARS-CoV-2 infection in other mammals, including species that serve as natural coronavirus reservoirs. "Our data confirm that TMPRSS2 broadly promotes initial infection at the virus-host interface and influences the outcome of infection," summarized Dr. Brown. This insight underscores the potential for various mammalian species to influence the ongoing evolution of SARS-CoV-2, highlighting the need for a broader perspective on viral transmission and mutation dynamics.
Innate Immune Activation and Cytopathology
The study revealed that TMPRSS2-mediated SARS-CoV-2 uptake boosts innate immune activation, which might help the host clear the virus. However, this same process increases virus-induced cytopathology, selecting for variants with enhanced immune evasion properties. This dual impact highlights a complex balance between viral uptake efficiency, immune response, and the resulting pathology.
The research found variant-specific differences in TMPRSS2 dependency, impacting immune activation, cytotoxicity, and virion secretion. For example, while all tested SARS-CoV-2 variants exhibited increased entry efficiency with TMPRSS2, the Omicron variant showed the least dependency. This suggests that different viral strains have evolved distinct strategies to optimize their entry and replication processes, potentially affecting clinical outcomes.
Mechanistic Insights: Entry and Replication Dynamics
The researchers used advanced techniques to compare viral entry into cells expressing either ACE2 alone or ACE2 with TMPRSS2. They observed that TMPRSS2 expression enhances early rates of virus replication and secretion, with notable differences among variants. For instance, the B.1 variant showed the most significant enhancement in viral replication and transcription rates with TMPRSS2, followed by the B.1.617.2 variant, while the Omicron variant displayed the least dependency on TMPRSS2.
Interestingly, the study found that TMPRSS2 not only boosts early viral replication but also modulates the kinetics of virus production and infected cell lifespan. Enhanced uptake led to higher rates of apoptosis and necrosis, especially in highly permissive cells expressing both ACE2 and TMPRSS2. This resulted in distinct virus production profiles, varying between cell types and viral variants.
Evolutionary Conservation and Cross-Species Analysis
The team extended their research to analyze TMPRSS2 orthologues from diverse mammalian species, including proposed zoonotic reservoirs and experimental models. They found that TMPRSS2 orthologues from different species could enhance SARS-CoV-2 entry, although with varying efficiency. This suggests a conserved mechanism across mammals, potentially facilitating the virus’s spread among different hosts.
Phylogenetic analysis showed evolutionary conservation of critical TMPRSS2 domains necessary for viral spike cleavage, indicating that these key functional elements have been maintained across species. This conservation supports the idea that TMPRSS2 plays a fundamental role in SARS-CoV-2 biology, not just in humans but also in a broader ecological context.
Conclusion: New Avenues for Research and Therapeutics
This groundbreaking study unveils new dimensions of SARS-CoV-2 entry mechanisms, highlighting the pivotal role of TMPRSS2 in facilitating viral uptake and shaping host immune responses. The discovery that TMPRSS2-mediated entry involves endosomes opens new research avenues to explore the detailed pathways and molecular interactions involved.
Understanding the diverse impacts of TMPRSS2 across different SARS-CoV-2 variants and mammalian species could inform the development of targeted antiviral therapies. By potentially blocking TMPRSS2 function or its interaction with the virus, new therapeutic strategies might emerge to curb SARS-CoV-2 transmission and mitigate severe disease outcomes.
As the world continues to grapple with COVID-19, this research underscores the importance of ongoing scientific inquiry to unravel the complexities of viral infection and evolution, paving the way for more effective interventions and a deeper understanding of pandemic dynamics.
The study findings were published in the peer reviewed journal: PNAS.
https://www.pnas.org/doi/full/10.1073/pnas.2407437121
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/study-finds-that-both-bacterial-cytolethal-distending-toxin-and-sars-cov-2-uses-cellugyrin-synaptogyrin-2-dependent-pathways-to-gain-cell-entry
https://www.thailandmedical.news/news/oklahoma-study-in-2023-finds-that-host-gene-snhg15-aids-sars-cov-2-entry-via-oncogene-rabl2a
https://www.thailandmedical.news/news/various-entry-receptors-for-coronaviruses-including-sars-cov-2-discovered-in-the-oral-cavity-which-exhibits-high-susceptibility-for-infections
