BREAKING Glaucoma News! Scientists At University Of California Regenerate Human Retinal Ganglion Cells By Manipulating Gene Regulation!
Nikhil Prasad Fact checked by:Thailand Medical News Team Sep 30, 2023 2 years, 2 months, 1 week, 6 days, 7 hours, 48 minutes ago
Glaucoma News: In recent groundbreaking news, researchers at the University of California have made an astounding discovery in the realm of eye health. They have successfully manipulated gene regulation to regenerate human retinal ganglion cells (RGCs). This achievement carries immense significance for individuals suffering from optic neuropathies, including glaucoma, which often lead to the death of these crucial cells.
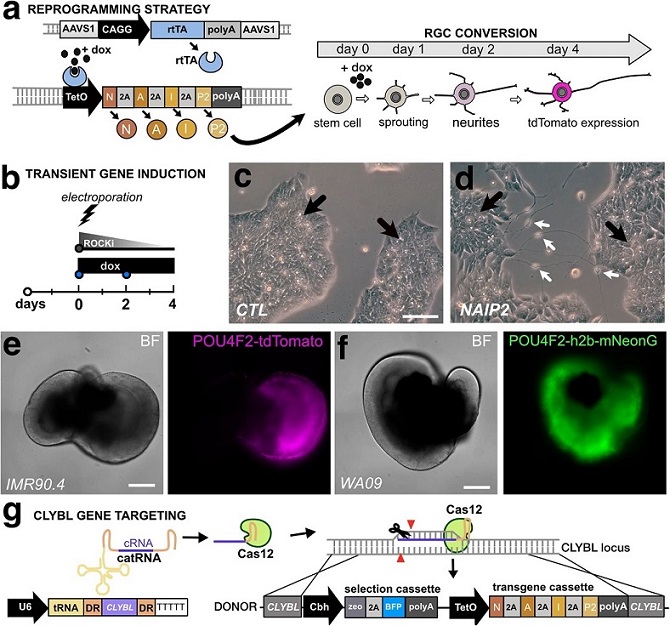 A conceptual diagram for a 3G Tet-ON system where doxycycline binds to constitutively expressed rtTA to induce polycistronic expression of NEUROG2, ATOH7, ISLET1, and POU4F2 (NAIP2) leading to conversion of RGC-like neurons. b Electroporation approach for the transient transfection of the NAIP2 transgene package. c PSCs that were transiently transfected with control (empty) or d NAIP2 plasmids and treated with dox for 3 days. Black arrows = PSCs, white arrows = converted neurons. Brightfield and fluorescent images of e the POU4F2-tdTomato+ reporter in a 66-day-old retinal organoid in the IMR90.4 genetic background and f a 65-day-old POU4F2-h2b-mNeonGreen+ retinal organoid in the WA09 genetic background. g U6 promoter-driven expression of an AsCas12a cRNA targeting the CLYBL safe harbor site for insertion of a zeocin selectable Tet-inducible transgene cassette. Scale c, d = 100 µm, e, f = 400 µm.
A conceptual diagram for a 3G Tet-ON system where doxycycline binds to constitutively expressed rtTA to induce polycistronic expression of NEUROG2, ATOH7, ISLET1, and POU4F2 (NAIP2) leading to conversion of RGC-like neurons. b Electroporation approach for the transient transfection of the NAIP2 transgene package. c PSCs that were transiently transfected with control (empty) or d NAIP2 plasmids and treated with dox for 3 days. Black arrows = PSCs, white arrows = converted neurons. Brightfield and fluorescent images of e the POU4F2-tdTomato+ reporter in a 66-day-old retinal organoid in the IMR90.4 genetic background and f a 65-day-old POU4F2-h2b-mNeonGreen+ retinal organoid in the WA09 genetic background. g U6 promoter-driven expression of an AsCas12a cRNA targeting the CLYBL safe harbor site for insertion of a zeocin selectable Tet-inducible transgene cassette. Scale c, d = 100 µm, e, f = 400 µm.
Glaucoma is a prominent cause of irreversible blindness, and it results from the deterioration of retinal ganglion cells, primarily due to axonal injury. The conventional approaches to address this issue involve cell transplantation and the stimulation of endogenous regeneration. However, the success of these strategies depends on understanding the intricate developmental programs necessary for their effectiveness.
To delve into this complex issue, the study team lead by Dr Devansh Agarwal from the Viterbi Family Department of Ophthalmology & the Shiley Eye Institute-UC San Diego, embarked on a journey to explore cellular reprogramming using transcription factor (TF) regulators associated with RGC development. These regulators were ingeniously integrated into human pluripotent stem cells (PSCs) as inducible gene cassettes. The pivotal discovery was made when the pioneer factor NEUROG2 was combined with other TFs, including ATOH7, ISL1, and POU4F2. The result was a significant conversion of cells into RGC-like induced neurons (RGC-iNs) within just under a week, by carefully pre-patterning with BMP inhibition.
Dr Agarwal told
Glaucoma News reporters at TMN, “Interestingly, the approximate 5 days that it took to generate RGC-iNs was markedly faster than other approaches that generally take upwards of 3–4 weeks, but as little as 15 days, albeit with much lower efficiency. It is worth noting that our NAIP2 cassette was designed based on a candidate approach from existing literature, and it is possible that other TF combinations might be as effective for RGC conversion. For instance, in mice Ascl1, Pou4f2, Islet1, and Atoh1 together were recently demonstrated to direct Müller cell conversion into RGCs1. For future endogenous repair efforts, other TF combinations may need to b
e explored to maximize conversion efficiency.”
These newly generated RGC-iNs displayed transcriptional profiles akin to authentic RGCs and exhibited electrophysiological properties, including AMPA-mediated synaptic transmission. Additionally, the researchers demonstrated that specific small molecules could inhibit neuronal death in two distinct pharmacological axon injury models. This dual approach, combining developmental patterning with RGC-specific TFs, offers invaluable insights into strategies for cell replacement and neuroprotection.
Understanding the Challenge
To appreciate the significance of this breakthrough, it's essential to grasp the challenges posed by conditions like glaucoma and the potential of retinal regeneration.
Optic neuropathies, which include glaucoma, are marked by the degeneration of retinal ganglion cells (RGCs) due to axonal injury. This damage results in vision impairment and, in severe cases, blindness. Scientists have explored various approaches to tackle this issue, including cell transplantation and stimulating the body's natural regenerative mechanisms. However, the success of these methods relies on understanding the intricate developmental programs necessary for their effectiveness.
While some animals, like zebrafish, possess remarkable regenerative capabilities for RGCs, mammals, including humans, face significant limitations in this regard.
Achieving endogenous regeneration of RGCs necessitates precise control over the timing and composition of developmental transcription factors (TFs). A deeper understanding of these factors could pave the way for innovative strategies to generate transplant-ready RGCs, promote endogenous repair, and provide a platform for evaluating neuroprotective treatments.
Unraveling the Role of Transcription Factors (TFs)
In the intricate landscape of the central nervous system (CNS) development, a multitude of cells emerge through the coordinated action of transcription factors (TFs). Early in development, morphogens play a vital role in regulating genes like Lhx2, Pax6, Rax, Six3, and Six6, which in turn govern eye and forebrain development. Bone Morphogenetic Protein 4 (Bmp4), a critical factor for retina specification, activates Sox2, while Bmp7 activates Pax6. BMP4 also influences dorsal patterning, and Sonic hedgehog (Shh) collaborates with SIX3 to specify ventral forebrain and neural retina.
As development progresses, TFs like NEUROG2, ATOH7, ISL1, and ASCL1 take center stage, regulating cell-cycle exit, RGC development, differentiation, and cell survival. NEUROG2 alone has the power to trigger neurogenesis, cell specification, differentiation, and migration. In chickens and mice, it teams up with Atoh7 to generate immature RGCs from cultured retinal pigment epithelial (RPE) cells. Isl1 and Pou4f2 (Brn3b) play pivotal roles in RGC differentiation, with their absence leading to various issues, including optic nerve hypoplasia and cell death. In mice, Atoh7 and Pou4f2 can even facilitate the transformation of Müller cells into RGCs.
The Sox superfamily, specifically the SoxC subfamily, which includes Sox4, -11, and -12, influences directional axonal growth and the formation of contralateral RGC axons. They also counteract Hes5, a suppressor of RGC differentiation. In essence, these TFs orchestrate the intricate dance of neural development.
Current Approaches and Challenges in RGC Generation
Methods for generating RGCs from human pluripotent stem cells (PSCs) do exist, but they are relatively slow and require immunopanning to achieve high purity. TFs, when used alone or in combination with small molecules, can initiate neural specification, but there's room for fine-tuning these combinations to expedite RGC production. This is where the research from the University of California comes into play.
Researchers at the university engineered human PSCs with doxycycline-inducible NEUROG2, ATOH7, ISL1, and POU4F2 transgene cassettes. NEUROG2 promotes general neurogenesis, while ATOH7, ISL1, and POU4F2 drive RGC differentiation. The critical discovery was that BMP signaling could be effectively blocked using a small molecule called LDN-193189 (LDN). When combined with TFs, LDN facilitated the rapid and efficient formation of RGC-like induced neurons (RGC-iNs), characterized by their long, branched neurites and POU4F2-tdTomato reporter expression in up to 94% of cells. Transcriptomic analyses and electrophysiological studies confirmed that RGC-iNs bore a striking resemblance to authentic RGCs.
Promising Insights and Future Directions
While the approximate five-day timeframe for generating RGC-iNs represents a significant leap in efficiency compared to other methods, the researchers acknowledge that there is room for further exploration. For instance, other TF combinations may prove equally effective or even more so in driving RGC conversion. The quest for maximizing conversion efficiency and specificity continues.
Despite the high efficiency achieved in generating RGC-iNs, not all cells converted into tdTomato+ neurons. This phenotypic heterogeneity may be attributed to gene silencing, which could affect some cells more than others. Finding methods to prevent gene silencing, increase protein expression, and provide temporal control over individual TFs could be a promising avenue for future research.
Intriguingly, while mice exhibit over 30 distinct RGC subtypes, human and primate RGCs display less diversity. This raises questions about the classification of RGC-iNs. Unlike adult human RGCs, which can be categorized into specific subtypes based on function and morphology, RGC-iNs did not readily segregate into such distinct groups. This suggests that RGC-iNs may require additional time or specific environmental factors to mature fully. It might also necessitate further modifications in the composition of TF transgene cassettes to align with the characteristics of specific RGC subtypes.
Conclusion
In conclusion, the researchers at the University of California have achieved a monumental breakthrough in regenerating human retinal ganglion cells through the manipulation of gene regulation. This discovery offers hope to millions of individuals suffering from optic neuropathies, particularly glaucoma, and other conditions leading to RGC death.
The combination of transcription factor reprogramming and chemical patterning has proven to be a highly effective approach for generating RGC-like cells. These cells not only mimic the transcriptional profiles and electrophysiological properties of authentic RGCs but also respond to neuroprotective compounds, offering new avenues for studies in RGC development and neuroprotection.
The potential applications of this research are vast, ranging from cell replacement therapies to the development of neuroprotective drugs. Moreover, this breakthrough opens the door to further exploration of TF combinations, gene silencing prevention, and the fine-tuning of techniques to generate specific RGC subtypes. The usage of phytochemicals to manipulate gene regulation and epigenetic changes to favor regenerating RGC-iNs is an area that Thailand Medical News is currently focusing on.
The study findings were published in the peer reviewed journal: NPJ Regenerative Medicine
https://www.nature.com/articles/s41536-023-00327-x
For the latest
Glaucoma News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/glaucoma-news-pou3f1-identified-as-a-regulator-of-contralateral-retinal-ganglion-cells-transcription-a-breakthrough-in-optic-nerve-regeneration
