BREAKING! Hidden Spike Cleavage Site in COVID-19 Virus Found to Supercharge Fusion and Possibly Boost Infectivity
Nikhil Prasad Fact checked by:Thailand Medical News Team Apr 08, 2025 4 days, 17 hours, 10 minutes ago
Medical News:
American Scientists Reveal the Critical Role of a Tiny Hidden Cut in the SARS-CoV-2 Virus Spike That Supercharges Its Ability to Infect Human Cells
A new study led by researchers from Boston Children’s Hospital, Harvard Medical School, the Institute for Protein Innovation, Dexorgen Inc., The Harvard Cryo-EM Center for Structural Biology, and Georgetown University has uncovered a crucial detail about how the COVID-19 virus enters human cells. Their work centers on a tiny but essential part of the virus's spike protein - called the S2’ cleavage site - that plays a powerful role in triggering the virus’s fusion with human cells.
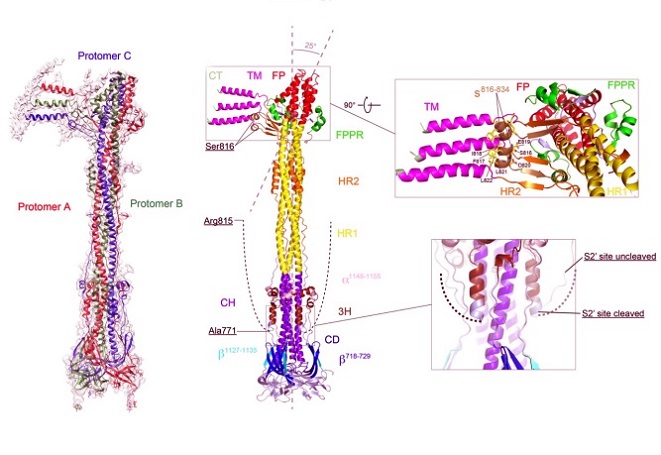
Structure of the S2’ fragment of SARS-CoV-2 spike in the postfusion conformation.
The structure of the S2’ trimer fits into a 3.1Å density map. Three protomers are colored in green, blue and red, respectively. Overall structure of the S2’ trimer in the postfusion conformation shown in ribbon diagram. Various structural components in the color scheme shown in B include b718-729, a b-strand formed by residues 718-729 in the S1/S2-S2’ fragment; 3H, threehelix segment; s816-834, a segment containing residues 816-834; FPPR, fusion peptide proximal region; FP, the fusion peptide; HR1, heptad repeat 1; CH, central helix region; CD, connectordomain; b1127-1135, a b-strand formed by residues 1127-1135; a1148-1155, an a-helix formed by residues 1148-1155; HR2, heptad repeat 2; TM, transmembrane anchor; and CT, cytoplasmic tail. The segment immediately upstream of the S2’ cleavage site (residues 772-815) is disordered and indicated by a dashed line. A close-up view of the transmembrane region after a 90° rotation is also shown and the residues of the structured s816-834 segment are indicated. Superposition of the C-terminal region of 3H from the S2’ trimer (colored) and the uncleaved S2 trimer (gray) is shown with an extra helical turn packing against the CH coiled-coil in the S2’ trimer.
Using advanced cryo-electron microscopy, the research team examined what happens after this S2’ site is cleaved by a host cell enzyme. What they found was both unexpected and revealing. This
Medical News report uncovers how this hidden step, often overlooked in many earlier studies, actually helps speed up the fusion process between the virus and human cells - potentially making the virus more infectious.
The Hidden Spike Cut That Turns the Virus into a Cell Fusing Machine
SARS-CoV-2, the virus that causes COVID-19, relies on a spike protein to invade human cells. This spike undergoes two cuts - first at a spot between its S1 and S2 regions (the S1/S2 site), and second at the S2’ site. While the first cut has been studied in detail, this study focuses on the lesser-known second cut, which is made by an enzyme like TMPRSS2 or trypsin.
To probe the effects of this cleavage, the team recreated the S2 fragment in lab cells, then used trypsin to simulate the cleavage at the S2’ site. The resulting protein structure rev
ealed that, although the location of the fusion peptide (the part that penetrates the host cell membrane) does not change, the S2’ cleavage helps arrange the spike protein into a form that can fuse much faster with the host cell membrane.
What they observed was a unique “intermediate” structure that appeared just before full membrane fusion - a rod-like trimer of spike proteins forming asymmetrical clusters. This structure likely plays a key role in reducing the time and energy needed for the virus to latch onto and enter human cells.
Cutting the Spike Supercharges Infection but Is Not Always Necessary
Interestingly, the study also found that this second cleavage - while it dramatically speeds up the process - is not absolutely required for the virus to infect cells. Even when the key amino acid at the cleavage site (Arginine 815) was replaced with other residues that block the cut, the virus could still infect human cells, albeit less efficiently.
In experiments using virus-like particles (VLPs), mutations at the S2’ site didn’t always eliminate infectivity. However, these mutated versions did not benefit from the same rapid entry advantage provided by TMPRSS2, the enzyme that normally performs the S2’ cleavage. This suggests that while the virus can bypass the need for this cut, it becomes far more efficient when it happens.
Moreover, this S2’ site may also help stabilize certain membrane fusion intermediates - temporary but essential shapes the virus must take to merge with a human cell. These stabilized structures could allow the virus to complete the infection process more rapidly, increasing its chances of success in hostile environments like the respiratory tract.
Potential Targets for Universal COVID Vaccines and Antiviral Drugs
One of the most exciting implications of this study is that the S2’ site might be a valuable target for future COVID-19 treatments and vaccines. Some broadly neutralizing antibodies already target this region. Since the S2’ cleavage alters the structure of the spike protein in ways that are critical for infection, stopping it could be a powerful strategy to neutralize the virus.
Importantly, the researchers found that after cleavage, the S2’ site epitope becomes less accessible, which could explain why some antibodies lose effectiveness during certain stages of infection. Using uncleaved versions of the spike protein in vaccines could potentially train the immune system to better recognize and attack this vulnerable spot.
Conclusions
The study shines new light on a previously underappreciated step in the infection cycle of SARS-CoV-2. By focusing on the S2’ site cleavage, the researchers demonstrated that this tiny molecular cut acts as a trigger that supercharges the fusion of the virus with human cells. While not absolutely required for infection, the cleavage dramatically accelerates the process, giving the virus a competitive edge. This newfound understanding not only helps decode how the virus operates but also opens new doors for the development of universal COVID vaccines and targeted therapies. The S2’ site and its surrounding structures are now recognized as critical hot spots for future intervention against current and emerging coronaviruses.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2025.04.02.646838v1
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/sars-cov-2-proteins-can-form-neurotoxic-amyloid-assemblies-that-are-taken-up-by-host-neurons
https://www.thailandmedical.news/news/sars-cov-2-e-protein-induces-long-term-immune-dysfunction
https://www.thailandmedical.news/news/why-your-immune-system-doesn-t-remember-covid-19-well-new-findings-on-cd4-t-cells
https://www.thailandmedical.news/articles/coronavirus
https://www.thailandmedical.news/pages/thailand_doctors_listings
