BREAKING! Latest COVID-19 News: European Research Discovers That C-Type Lectin Receptors In Immune Cells Facilitates SARS-CoV-2 Infection
Source: Latest COVID-19 News Aug 12, 2020 4 years, 8 months, 2 weeks, 2 hours, 25 minutes ago
Latest COVID-19 News: European researchers from Univ. Grenoble Alpes-France, Universidad Complutense School of Medicine-Spain and Universita` degli Studi di Milano-Italy have made a shocking discovery that a type of sugar-based receptor on innate immune cells called C-type lectin receptors promotes the entry of the SARS-CoV-2 coronavirus into other cells that carry the viral receptor, angiotensin-converting enzyme 2 (ACE2).
.jpg)
This new discovery has massive implications and finally shows how innate immune cells interact with the virus early to enhance its capture and the spread of infection. It also explains how this could lead to an unbalanced innate immune response.
The study has been released on a preprint server the last few hours and is currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2020.08.09.242917v1
It is well known that the cells of the human body communicate with each other in a plethora of ways.
The receptors found on innate immune cells ie the C- type Lectin Receptors (CLRs) is one such means of signaling, belonging to the category of pathogen recognition receptors (PRR). These are useful when innate immune cells called antigen presentation cells (APCs) need to pick up pathogen-associated molecular patterns (PAMPs), and when the normal immune response needs to be activated.
So far, there is a broad range of CLRs that has been identified depending on the cell type, including Dectin-2, DC-SIGN, and Langerin.
It is known that when C- type Lectin Receptors (CLRs) interact with their carbohydrate ligands on dendritic cells (DCs), one important type of APC, the immune response is either activated or modulated to allow tolerance.
Importantly this is achieved through antigen presentation in lymphoid organs, and secondarily via cytokine release.
Dendritic cells or DCs are found at sites where pathogens first come into contact with the body, such as epithelial surfaces of the upper respiratory tract and the lung alveoli.
Significantly one strategy of immune evasion or even harnessing immune activity for the benefit of the pathogen is to bind to CLRs on the surface of the DCs in order to facilitate their transfer towards their real host receptor and cell entry. CLRs, especially L-SIGN (or DC-SIGNR) or DC-SIGN, are well-known for this role in the entry of viruses like HIV, cytomegalovirus, dengue, Ebola, and Zika virus.
For example, the HIV virus binds to DC-SIGN, which allows the DC to be directly infected but also passes on the bound virus to T cells, the actual target cell for this virus. This is called cis and trans infection. These receptors also enhance the infection of host cells by SARS-CoV-1.
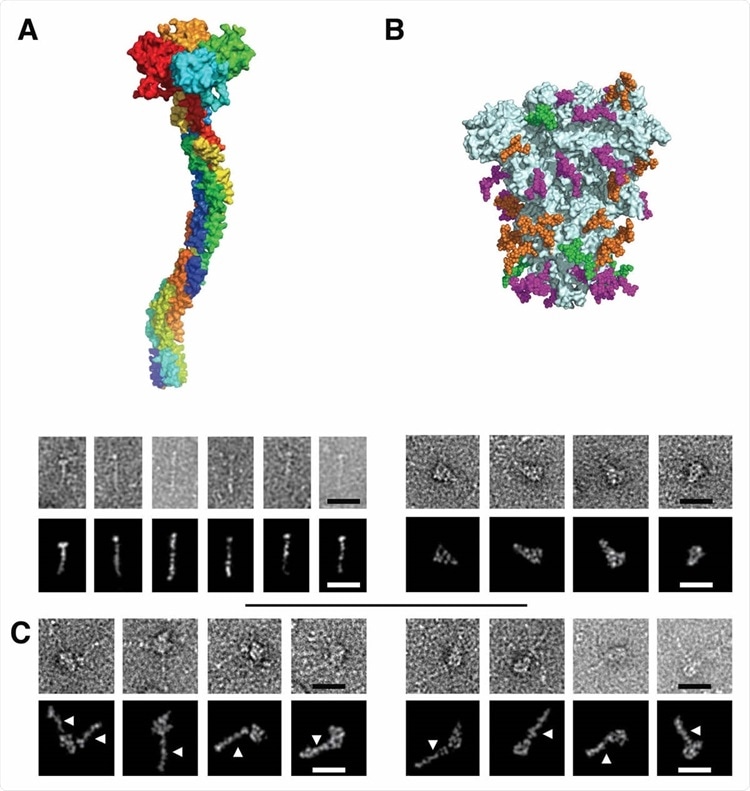 Electron Microscopy micrographs of DC-SIGN/S protein complexes (A) DC-SIGN. Top: model of DC-SIGN ECD tetramer adapted from Tabarani et al (2009). On the bottom: Negative staining images of DC-SIGN. Top row: origi
nal images; bottom row: Photoshop processed images. The scale bar represents 25 nm. (B) Spike protein. Top: model of the glycosylated Spike adapted from model of Casalino et al pdb 6vsb). Glycan sites are represented with color code derived from the work of Crispin et coll. (Watanabe et al., 2020a), according to oligomannose-type glycan content, in green (80-100%), orange (30-79%) and magenta (0-29%). On the bottom: Negative staining images of spike protein. Top row: original images, bottom row: Photoshop processed images. The scale bar represents 25 nm. (C) Complex between DC-SIGN and spike protein. Negative staining image of the complexes between DC-SIGN and spike protein. The white arrows highlight DC-SIGN molecules. Top row: original images; bottom row: Photoshop processed images. The scale bar represents 25 nm.
Electron Microscopy micrographs of DC-SIGN/S protein complexes (A) DC-SIGN. Top: model of DC-SIGN ECD tetramer adapted from Tabarani et al (2009). On the bottom: Negative staining images of DC-SIGN. Top row: origi
nal images; bottom row: Photoshop processed images. The scale bar represents 25 nm. (B) Spike protein. Top: model of the glycosylated Spike adapted from model of Casalino et al pdb 6vsb). Glycan sites are represented with color code derived from the work of Crispin et coll. (Watanabe et al., 2020a), according to oligomannose-type glycan content, in green (80-100%), orange (30-79%) and magenta (0-29%). On the bottom: Negative staining images of spike protein. Top row: original images, bottom row: Photoshop processed images. The scale bar represents 25 nm. (C) Complex between DC-SIGN and spike protein. Negative staining image of the complexes between DC-SIGN and spike protein. The white arrows highlight DC-SIGN molecules. Top row: original images; bottom row: Photoshop processed images. The scale bar represents 25 nm.
Typically coronaviruses are characterized by the presence of numerous spikes, with the surface of each spike head and stalk being covered by glycans to the tune of 60% to 90%, respectively.
These sugar based glycans are mostly conserved between the two SARS-CoVs, in chemical composition, and in position. This has been suggested to create a glycan shield against neutralizing antibodies. Earlier studies using molecular dynamic simulations have also suspected possible mechanisms via the spike glycans can modulate ACE2-virus interactions by stabilizing the receptor-binding domain (RBD) in the up conformation.
Significantly yet another possible method of interaction is that the spike sugars may anchor the coronavirus to CLRs on the host cells since almost 30% are oligomannose sugars that are ligands for CLRs. Some mutations that affect the virulence of the protein are known to affect the level of glycosylation of the spike.
The stud team focused on the interactions between the novel coronavirus and these receptors.
The team found that that C- type Lectin Receptors or CLRs bind multiple sites on the S protein, and this initial adhesion to the host cells is essential for efficient capture, the concentration of virus particles on the host cell surface, and then searching for and attaching to the ACE2 receptor.
Professor Dr Rafael Delgado from the department of biomolecular research at the Universidad Complutense School of Medicine-Spain, who is also the corresponding author of the study explained to Thailand Medical News via a phone interview, “Although such additional receptors may not promote any fusion step, they can drive viral internalization through endocytic processes or simply by viral adhesion to the host cell, accumulation of viral particles on the cell surface and finally engagement with the primary receptor followed by the fusion event.”
The researchers also went on to examine the role of the CLRs DS-DIGN and L-SIGN in SARS-CoV-2 pseudovirion infection.
Pseudovirions are synthetic viruses used to inject genetic material, including DNA and RNA, with specific and desired traits into bacterial and eukaryotic cells.
For the study, the host cell used was monocyte-derived DCs (MDDCs) and M2 monocyte-derived macrophages (M2-MDM), which express DC-SIGN. They found that despite the efficient infection of these cells by the pseudovirions, the binding was not via these CLRs since it was not blocked by anti-DC-SIGN antibodies.
Interestingly, when the pseudovirions were incubated with MDDCs and then placed on Vero cells, efficient trans-infection took place. This was significantly reduced by anti-DC-SIGN antibodies.
The study team next tested the binding of SARS-CoV-2 to T cells lacking ACE2 receptors and thus incapable of being directly infected. They found that when the pseudovirus was incubated with a mixture of these cells, both those which expressed and those which did not express the CLRs, with Vero cells, they found that the latter were efficiently transfected in the presence of the T cells with CLR expression. Again, this was significantly reduced by anti-DC-SIGN antibodies by 87% and 79%, respectively.
The team lastly utilized a known sugar that mimics the natural ligand of DC-SIGN. This compound, called PM26, inhibited the binding of DC-SIGN to the spike protein. It also prevented 99% and 77% of the transfection of Vero cells when incubated with a mixture of pseudovirions and pre-treated DCs.
The study also showed that the binding between these C- type Lectin Receptors or CLRs and the spike protein is similar to a “Velcro effect,” with a multitude of binding sites rather than just one.
It was observed however; L-SIGN and DC-SIGN both have micromolar affinities for the spike protein, which will result in a stable surface affinity that is higher by several orders of magnitude at the cell surface. The microdomain organization of CLRs allows multiple attachment points to be presented for viral capture.
Importantly, DC-SIGN enhanced infection by SARS-CoV-2 pseudovirions significantly when the latter were incubated with cells bearing the CLR and then on ACE2-carrying Vero cells.
Dr Delgado commented, “This research shows that DC/L-SIGN are important enhancers of infection mediated by the S protein of SARS-CoV-2 that greatly facilitate viral transmission to susceptible cells. While DC-SIGN is found in immature DCs in the submucosa and in the tissue-resident macrophages, including the alveolar macrophages, L-SIGN is found in human type 2 alveolar cells and lung endothelium.”
The research findings show that the expression of CLRs like the above is modulated by IFN, TGF-β, and other anti-inflammatory molecules.
But when ligand binding occurs at the DC-SIGN or L-SIGN receptor, an immune response occurs with the release of pro-inflammatory cytokines like IL-6.
This what that has been found to contribute to the cytokine storm seen in severe COVID-19 with its often fatal termination.
Importantly, SARS-CoV-2 can also prevent the production of type I and type III interferons and thus upregulate the expression of these CLRs.
The research findings signals the importance of developing DC-SIGN and L-SIGN antagonists to reduce the severity of infection by inhibiting their activity or expression, just as PM26 does.
The PM26 can not only bind the lectin but also enhance its internalization, reducing the number of CLRs available for the virus to bind it and transfect susceptible cells.
Hence this in return could also induce a pro-inflammatory response on binding to DC-SIGN, which could help reduce the severity of the infection.
The study team says that this represents an interesting direction for further research.
Meanwhile the new research findings is creating a major stir among the research community over the last few hours as the implications are also numerous in other research areas.
For the
Latest COVID-19 News, keep on logging to Thailand Medical News.
.jpg)
