BREAKING! Leading Cardiologists And Doctors From Massachusetts Issue Warning Of Adverse Drug Interactions In COVID-19 Patients Treated With Paxlovid!
Source: COVID-19 Drugs - Paxlovid Oct 13, 2022 2 years, 6 months, 1 week, 8 hours, 2 minutes ago
COVID-19 Drugs: Leading cardiologists and physicians from various hospitals and medical centers across the state of Massachusetts-USA have issued a warning of adverse drug interactions especially involving heart medications in COVID-19 patients treated with Paxlovid (Nirmatrelvir/Ritonavir).
.jpg)
The team of specialists from Lahey Hospital and Medical Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts General Hospital, Beth Israel Deaconess Medical Center, Saint Vincent Hospital had found that the COVID-19 drug Paxlovid interacts with certain common heart medications and in some cases can even contribute to or cause fatal outcomes!
Paxlovid or nirmatrelvir-ritonavir (NMVr) was approved by the U.S. FDA under the Biden administration to treat symptomatic, non-hospitalized patients with coronavirus disease-2019 (COVID-19) who are at high risk of progression to severe disease.
Patients with cardiovascular risk factors and cardiovascular disease are at a high risk of developing adverse events from COVID-19 and as a result have a higher likelihood of receiving NMVr.
However, unknown to many, ritonavir, the pharmaceutical enhancer used in NMVr, is an inhibitor of the enzymes of CYP450 pathway, particularly CYP3A4 and to a lesser degree CYP2D6, and affects the P-glycoprotein pump.
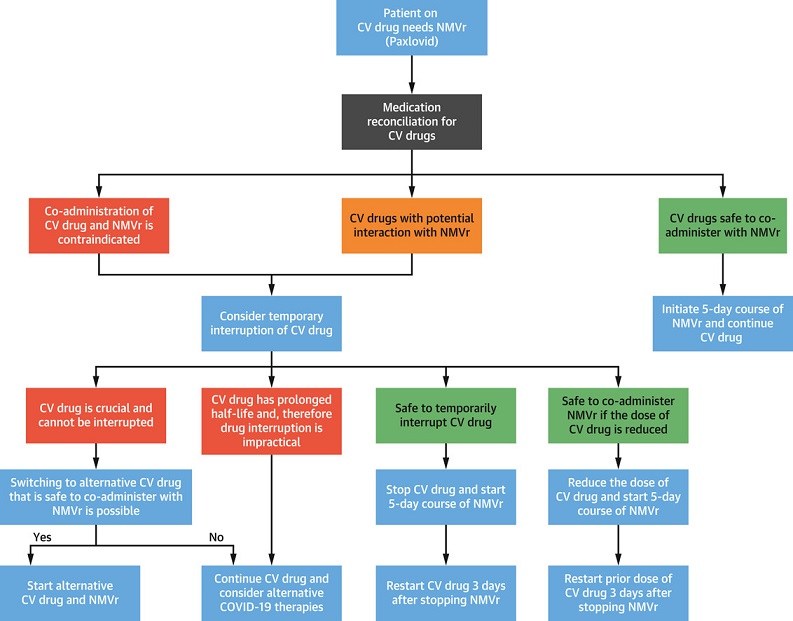
It was found that co-administration of NMVr with medications commonly used to manage cardiovascular conditions can potentially cause significant drug-drug interactions and may lead to severe adverse effects including in some cases deaths.
It is critical that both attending physicians and even patients or next of kins to be aware of such interactions and take appropriate measures to avoid them.
The study team in this review, list the potential drug-drug interactions between NMVr and commonly used cardiovascular medications based on their pharmacokinetics and pharmacodynamic properties.
The study review and warning were published in the peer reviewed Journal of the American College of Cardiology.
https://www.sciencedirect.com/science/article/abs/pii/S0735109722068255
The published review is the first to examine the potential drug-drug interactions (DDIs) between Paxlovid and commonly used cardiovascular medications, as well as potential options to mitigate severe adverse effects.
Senior author, Dr Sarju Ganatra, MD, director of the cardio-oncology program at Lahey Hospital and Medical Center in Burlington who is also regarded as being among one of the leading cardiologists in the United States told Thailand Medical News, "Awareness of the presence of drug-drug interactions of Paxlovid with common cardiovascular drugs is key. System-level interventions by integrating drug-drug interactions into electronic medical records could help avoid related adverse events."
He added, "The prescription of Paxlovid could be incorporated into an order set, which allows physicians, whether it be primary care physicians or cardiology providers, to consciously rule out any contraindications to the co-administration of Paxlovid. Consultation with other members of the health care team, particularly pharmacists, can prove to be extremely valuable. However, a health care provider's fundamen
tal understanding of the drug-drug interactions with cardiovascular medications is key."
Nirmatrelvir-ritonavir (NMVr), commonly known as Paxlovid received emergency use authorization from the U.S. Food and Drug Administration in December 2021 as an oral antiviral agent for the treatment of symptomatic, non-hospitalized adults with mild to moderate COVID-19 infection who are at high risk for progression to severe disease. Patients with heart disease and other risk factors, including diabetes, high blood pressure, chronic kidney disease and smoking make up a large portion of the high-risk population for whom Paxlovid is beneficial.
Although some have claimed that Paxlovid has been shown to be very effective in patients with existing heart disease, the study team warned that it has significant DDIs with commonly used cardiovascular medications, highlighting the importance for all clinicians to be familiar with these DDIs.
Surprisingly, at present there is limited clinical information regarding DDI-related adverse events even by the drug manufacturer or by the U.S. FDA!
The
COVID-19 Drugs study team utilized existing knowledge and data regarding how therapies like Paxlovid typically react with other medications to provide guidance regarding potential interactions and the associated likely consequences based on the degree of interaction.
The published review is the first that provides an in-depth overview of a variety of cardiovascular medications used to treat many forms of heart disease.
The study team listed the five of the most important cardiovascular drug interactions with Paxlovid to be aware of as follows:
-
Anti-Arrhythmic Agents Or Drugs
Typically, anti-arrhythmic agents are used to manage abnormal heart rhythm or arrhythmia. Many of these drugs are metabolized in a way that increases plasma levels when co-administered with Paxlovid. While it may be possible to start Paxlovid after 2-2.5-day temporary discontinuation of the anti-arrhythmic agents, this may not be feasible from a practical standpoint.
Attending clinicians are advised to consider alternative COVID-19 therapies and avoid co-administration of these agents with Paxlovid. Sotalol, another anti-arrhythmic agent, is renally cleared and does not interact with Paxlovid.
-
Anticoagulants And Antiplatelet Drugs
Blood thinners used to treat or prevent blood clots that are known as anticoagulants including warfarin may be co-administered with Paxlovid but require close monitoring of clotting factors in bloodwork. The plasma levels of all direct oral anticoagulants increase when co-administered with Paxlovid, therefore dose adjustment or temporary discontinuation and use of alternative anticoagulants may be required.
Often, antiplatelet agents are used for the treatment of coronary artery disease, particularly if a patient has received a stent. Aspirin and prasugrel are safe to co-administer with Paxlovid. However, it should be noted that there is an increased risk of blood clots when Paxlovid is given alongside clopidogrel and an increased risk of bleeding when given with ticagrelor. When possible, these agents should be switched to prasugrel. If patients have contraindication to taking prasugrel, then co-administration of Paxlovid should be avoided and alternative COVID-19 therapies should be considered.
-
Statins
It was found that co-administration of simvastatin or lovastatin with Paxlovid can lead to increased plasma levels and subsequent muscle weakness (myopathy) and rhabdomyolysis, a condition in which the breakdown of muscle tissue releases a damaging protein into the bloodstream. These agents should be stopped prior to initiation of Paxlovid. A dose reduction of atorvastatin and rosuvastatin is reasonable when co-administered with Paxlovid. The other statins are considered safe when given along with Paxlovid.
-
Ranolazine (Ranexa)
The study team found that plasma concentration of ranolazine, used to treat angina and other heart-related chest pain, is exponentially increased in the presence of CPY450 inhibitors like Paxlovid, thereby increasing the risk of clinically significant QT prolongation and torsade de pointes (a type of arrhythmia). Co-administration of Paxlovid is therefore contraindicated. Temporary discontinuation of ranolazine is advised if prescribing Paxlovid.
-Immunosuppressive agents
The team found that the plasma levels of immunosuppressive agents prescribed for patients who have undergone heart transplantation exponentially rise to toxic levels when co-administered with Paxlovid. Temporary reduction of dosing of immunosuppressive agents would require frequent monitoring and be logistically difficult. Therefore, alternative COVID-19 therapies should be considered in these patients.
The authors concluded that the awareness and availability of other COVID-19 therapies enable clinicians to offer alternative treatment options to patients who are unable to take Paxlovid due to DDIs.
The study team commented, “We have described the many known and potential DDIs between various cardiovascular medications and NMVr. The importance of medication reconciliation before initiation of NMVr cannot be overemphasized to avoid serious DDIs. Dose adjustment or discontinuation of cardiovascular medications may be required for the duration of NMVr treatment and 3-5 days after completion.”
Thailand
Medical News would like to add that all COVID-19 patients especially their next of kin should at all times monitor and document all treatment protocols and also all patient status and other records for future reference in case there is a need for legal suits. It has been found that in some cases, patients are not actually dying form COVID-19 but rather the drugs used or the wrong treatment protocols used by ignorant or misinformed doctors. Also, these documents can be used to sue the drug manufacturers, the regulatory agencies and also health authorities who design treatment protocols or guidelines.
It should also be noted that individuals already infected with the SARS-CoV-2 can develop cardiovascular issues including myocarditis and arrhythmia, in such a vulnerable condition usage of drugs that can further aggravate the heart should always be avoided especially drugs like fluvoxamine that is being advocated as a prophylactic and therapeutic agent for COVID-19 and Long COVID by some charlatans.
https://www.thailandmedical.news/news/world-health-organization-issues-warning-against-the-use-of-fluvoxamine-and-colchicine-to-treat-covid-19
https://www.thailandmedical.news/news/most-who-have-been-exposed-to-the-proteins-of-the-sars-cov-2-virus-will-have-shortened-lifespans-stop-using-fluvoxamine-for-ba-2-infections
For the latest on
COVID-19 Drugs, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/breaking-japanese-study-finds-u-s-fda-approved-and-promoted-covid-19-drug-remdesivir-induces-cardiomyocyte-dysfunction-and-is-cardiotoxic
https://www.thailandmedical.news/news/covid-19-drugs-study-published-in-journal-of-the-heart-rhythm-society-warns-that-remdesivir-can-cause-dangerously-low-heart-rate-in-covid-19-patients
https://www.thailandmedical.news/news/covid-19-drugs-university-of-cincinnati-study-warns-against-use-of-u-s-fda-approved-remdesivir-due-to-drug-interactions-and-increased-toxicity
https://www.thailandmedical.news/news/covid-19-antivirals-interim-who-solidarity-trial-results-confirms-that-remdesivir,-lopinavir-and-interferon-have-no-effect-on-covid-19-mortality
https://www.thailandmedical.news/news/covid-19-scams-who-issues-warning-against-use-of-remdesivir-for-covid-19--countries-should-beware-of-any-therapeutics-approved-in-the-usa
https://www.thailandmedical.news/news/molnupiravir--what-wrong-with-america-why-is-the-american-government-and-agencies-funding-and-pushing-potentially-toxic-drugs-to-treat-covid-19
https://www.thailandmedical.news/news/america-latest-trump-s-controlled-fda-approves-remdesivir-despite-who-and-other-studies-showing-ineffectiveness-in-treating-covid-19
https://www.thailandmedical.news/news/first-published-case-report-links-usage-of-molnupiravir-to-treat-covid-19-to-acute-renal-failure
https://www.thailandmedical.news/news/much-touted-merck-s-covid-19-drug-molnupiravir-shown-to-be-less-effective-in-new-analysis-experts-question-its-efficacy-against-the-omicron-variant
https://www.thailandmedical.news/news/georgia-state-university-researchers-try-to-peddle-mutagenic-drug-molnupiravir-as-possible-covid-19-therapeutic-after-study-on-ferrets
.jpg)
