BREAKING Medical Devices News! Some Philips MRI Machines Have Risk Of Exploding! U.S. FDA Initiates Class One Recall!
James Rosh Jan 03, 2024 1 year, 3 months, 3 weeks, 2 days, 15 hours, 40 minutes ago
Medical Devices: In a startling development within the realm of medical devices, the U.S. Food and Drug Administration (FDA) has recently issued a Class I recall for Philips North America's Panorama 1.0T HFO MRI systems. This drastic measure has been taken due to the potential risk of explosion associated with the use of these magnetic resonance imaging machines. The ramifications of this recall extend beyond the immediate implications for patients and healthcare providers, shedding light on the broader challenges and responsibilities inherent in the medical device industry.
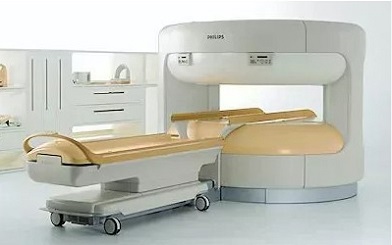 Philips Panorama 1.0T HFO MRI systems.
Recall Origins and Severity
Philips Panorama 1.0T HFO MRI systems.
Recall Origins and Severity
The U.S. FDA's decision to classify the recall as Class I underscores the gravity of the situation, signifying that the continued use of the Panorama 1.0T HFO MRI systems "may cause serious injuries or death." This categorization is reserved for situations where there is a substantial risk of severe consequences. The recall specifically targets 150 units of the Panorama 1.0T HFO MRI systems distributed in the U.S. between January 1, 2001, and October 1, 2016.
https://www.fda.gov/medical-devices/medical-device-recalls/philips-north-america-llc-recalls-panorama-10t-hfo-due-risk-explosion-during-quench-procedure-caused?utm_medium=email&utm_source=govdelivery
It is not known if Philips MRI machines being used in hospitals in other countries are also affected but relevant health authorities, medical device regulatory agencies and hospitals directors should also be looking into this urgently!
Some experts are also warning that patients and consumers should avoid hospitals using Philips MRI machines or
Medical Devices for the time being till there is proper assurance of safety. Patients have the right to ask hospitals what brand and models of diagnostic devices they are using to help them decide whether they want to use that particular hospital.
Thailand
Medical News strongly advices readers to only use hospitals that either use the Siemens, G.E. or Canon MRI or CT Scan machines as these machines are known for their safety and also accuracy.
According to the U.S. FDA, the primary concern prompting this unprecedented recall is the risk of explosion during a critical procedure known as a quench. This infrequent process involves the rapid release of evaporated helium through the venting system to maintain the superconductivity of the MRI magnet. The potential compromise of the venting system's integrity due to an unknown blockage during this process is the crux of the issue, leading to the he
ightened risk of chemical exposure, lack of oxygen, tissue damage, and other serious injuries.
Reported Incident and Historical Context
While there has been only one reported explosion in the 22 years of the system's use, the U.S. FDA has deemed it necessary to take decisive action, given the severity of potential incidents. The risks associated with the Panorama 1.0T HFO MRI systems extend beyond the immediate explosion, encompassing chemical exposure, lack of oxygen, tissue damage, and mechanical trauma from debris. The potential for life-altering consequences, including brain injury and death, necessitates the proactive approach taken by the FDA in initiating this recall.
Philips' Ongoing Safety Challenges
This recall is not an isolated incident for Philips. The company has been grappling with safety and quality concerns related to its medical devices, as evidenced by the recall of millions of sleep apnea devices and ventilators in 2021.
https://www.fda.gov/medical-devices/safety-communications/update-certain-philips-respironics-ventilators-bipap-machines-and-cpap-machines-recalled-due
https://www.thailandmedical.news/news/breaking-u-s-fda-sounds-alarm-on-recall-of-philips-respiratory-medical-devices-spanning-from-cpap,-bipap-and-mechanical-ventilator-devices
Additionally, a recent FDA notice highlighted reports of fire, smoke, and burns associated with users of Philips' Dreamstation 2 continuous positive airway pressure machines.
https://www.fda.gov/medical-devices/safety-communications/carefully-monitor-philips-dreamstation-2-cpap-machines-signs-overheating-fda-safety-communication
The accumulation of safety alerts raises questions about the effectiveness of Philips' quality control measures and the potential impact on its standing within the medical device market.
Philips' Immediate Response and Mitigation Measures
In response to the FDA's Class I recall, Philips has swiftly taken action to mitigate the risks associated with the Panorama 1.0T HFO MRI systems. Healthcare providers in possession of the affected units have been urged to cease using them immediately. Philips has provided detailed instructions for the proper disposition of the devices, emphasizing that a manual quench of the magnet should only be performed in emergency situations.
Furthermore, healthcare organizations have been directed to prominently post a "do not use" notice near the impacted MR systems, alerting all users to the product issue and associated hazards. This proactive approach is crucial in preventing any inadvertent use of the affected MRI systems and ensuring that both patients and medical staff are aware of the potential dangers.
Broader Implications for Healthcare Providers
The urgent nature of this Class I recall underscores the importance of healthcare providers' immediate compliance with the prescribed measures. The potential risks associated with the continued use of the Panorama 1.0T HFO MRI systems demand a swift response to safeguard patient well-being and prevent any catastrophic incidents within medical facilities.
Beyond the immediate concerns, healthcare providers are prompted to reassess their protocols for monitoring and evaluating the safety of medical devices regularly. This incident serves as a stark reminder that even well-established and widely used technologies can pose unexpected risks, necessitating constant vigilance and proactive measures to ensure patient safety.
Conclusion
The FDA's Class I recall of the Philips Panorama 1.0T HFO MRI systems due to the risk of explosion is a watershed moment that brings into focus the critical intersection of technological advancements and patient safety within the medical device industry. As healthcare providers navigate the immediate challenges posed by the recall, there is an overarching need for the industry to reevaluate its safety protocols, quality assurance measures, and communication strategies to maintain public trust.
The incident serves as a call to action for regulatory bodies, medical device manufacturers, and healthcare providers to collaborate more closely in ensuring the safety and efficacy of medical technologies. The repercussions of this recall extend far beyond the affected MRI systems, challenging the industry to adopt a proactive stance in addressing potential risks associated with evolving medical technologies. In an era of rapid innovation, prioritizing patient safety must remain at the forefront of the medical device industry's mission.
Instead of spending monies on sponsored post in certain garbage mainstream media, Philips should be spending the monies to ensure proper safety for users of its medical devices!
For the latest on
Medical Devices, keep on logging to Thailand Medical News.
