BREAKING Medical News! Methoxy-Mycolic Acid From BCG Shows Promise In Relieving Monocyte Exhaustion And Promoting T Cell Proliferation!
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 19, 2024 1 year, 3 months, 1 week, 17 hours, 7 minutes ago
Medical News: In the perpetual pursuit of effective treatments for sepsis, a recent breakthrough study conducted at Virginia Tech in Blacksburg, USA, has revealed promising results in the use of Methoxy-Mycolic Acid (M-MA), a derivative of Mycobacterium Bovis Bacillus Calmette - Guérin (BCG). The research, which focuses on addressing monocyte exhaustion, a significant factor in sepsis-related complications, brings renewed hope for improved therapeutic interventions in critical care settings. The findings might even be useful in the context of COVId-19 treatments.
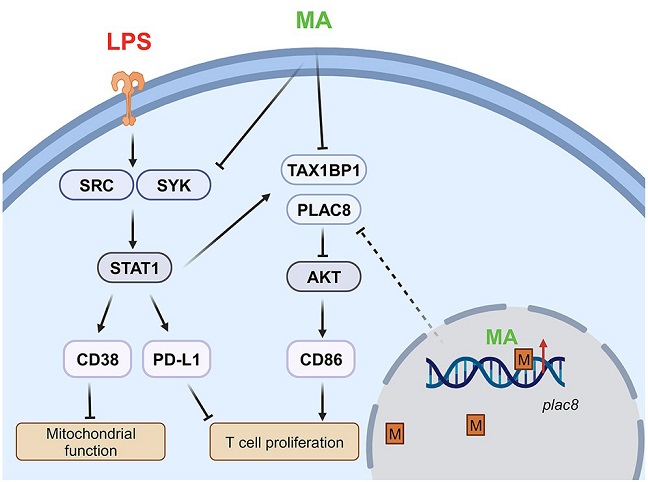 Graphical Abstract Of Study Showing That Methoxy-Mycolic Acid From BCG Relieves Monocyte Exhaustion
Graphical Abstract Of Study Showing That Methoxy-Mycolic Acid From BCG Relieves Monocyte Exhaustion
& Promotes T Cell Proliferation
Sepsis, characterized by a dysregulated immune response to infections or injuries, remains a leading cause of mortality and morbidity worldwide. The intricate interplay of sustained pathogenic inflammation and immune suppression often leads to multi-organ dysfunction and an increased vulnerability to secondary infections. Monocytes, key players in the immune system, can undergo a state of exhaustion during sepsis, marked by a shift in the expression of various inflammatory and immune-regulatory molecules. Despite the urgency for effective treatments, current strategies to combat sepsis-induced monocyte exhaustion are limited.
BCG, well-known for its role as a tuberculosis vaccine, has garnered attention for its potential cross-protective effects against a spectrum of pathogens. The study covered in this
Medical News report, dives into the specific properties of Methoxy-Mycolic Acid, aiming to understand its efficacy in mitigating monocyte exhaustion and restoring immune homeostasis.
Exploring the Key Findings
-
M-MA Alleviates Monocyte Exhaustion
The study demonstrates that M-MA effectively blocks the expansion of exhausted monocyte populations induced by lipopolysaccharide (LPS) challenges, a common model for simulating septic conditions. This critical finding suggests that M-MA holds the potential to restore immune balance in the face of prolonged inflammatory challenges.
In more granular detail, the co-treatment with M-MA resulted in a reduction in the expression of two pivotal molecules, CD38 and PD-L1, both associated with pathogenic inflammation. Simultaneously, M-MA restored the expression of CD86, an immune-enhancing mediator. This triad of effects indicates a shift towards a more balanced and regulated immune response, offering a potential therapeutic avenue for managing sepsis-induced monocyte exhaustion.
-Mitochondrial Function and T Cell Proliferation
Another compelling aspect of M-MA's efficacy lies in its ability to restore mitochondrial function in exhausted monocytes. Oxidative stress, often a consequence of prolonged inflammatory challenges, can compromise mitochondrial integrity. The study reveals that M-MA not only mitigated this oxidative stress but also demonstrated the potential to enhance the support pro
vided by monocytes to T cell proliferation.
The interconnectedness between monocytes and T cells is crucial for mounting an effective immune response. By addressing mitochondrial dysfunction in exhausted monocytes, M-MA showcases a multifaceted impact on immune function, providing a foundation for potential therapeutic applications.
-Molecular Mechanisms
Unraveling the molecular intricacies, the study delves into the signaling circuitries modulated by M-MA. One key observation is the reduction in Src-STAT1-mediated inflammatory polarization, a pathway implicated in prolonged immune activation during sepsis. By intervening in this signaling cascade, M-MA demonstrates its potential to curb excessive inflammation, a hallmark of septic conditions.
Furthermore, M-MA's impact extends to the reduction in the production of immune suppressors TAX1BP1 and PLAC8. These molecules play a role in autophagy-mediated suppression of key immune-enhancing pathways, and their inhibition by M-MA presents a promising avenue for restoring immune functionality.
-Epigenetic Landscape
The study takes a significant step forward by exploring the epigenetic dimension of monocyte exhaustion. DNA methylation, a stable imprint on the genome, was found to be significantly altered in exhausted monocytes. M-MA showcased its ability to erase this methylation memory, particularly restoring methylation at the Plac8 gene.
Genome-wide methylation analyses highlighted not only the global impact of M-MA but also the specificity of its effects on key regulatory regions. The potential to modulate the epigenetic landscape opens new horizons for understanding and intervening in the long-term effects of immune exhaustion.
-TREM2-Independence and Synergy with TRAM Deletion
Addressing concerns about potential dependencies on specific receptors, the study demonstrates that M-MA's effects in restoring monocyte homeostasis are independent of the cell-surface receptor TREM2. This adds a layer of versatility to M-MA's applicability across diverse immune contexts.
Interestingly, the study also explores the synergy between M-MA and TRAM deletion, a component of the Toll-like receptor 4 (TLR4) signaling pathway. TRAM deletion alone was previously shown to inhibit monocyte exhaustion, and the addition of M-MA further enhanced the restoration of monocyte functionality.
This synergy points towards potential combinatory approaches in treating sepsis-related immune dysregulation.
Implications and Future Directions
The groundbreaking findings presented in this study lay the foundation for a paradigm shift in sepsis therapeutics. M-MA's ability to modulate key molecular pathways, restore mitochondrial function, and re-balance the genomic methylation landscape provides a comprehensive understanding of its mechanism of action.
As the research community embarks on further investigations, the focus should extend to assessing the pharmacodynamics and pharmacokinetics of M-MA in animal models. Understanding the compound's behavior in vivo is essential for progressing towards clinical trials and, ultimately, practical applications in critical care settings.
Moreover, exploring the potential of M-MA in combination with existing sepsis treatments could offer a synergistic approach, enhancing overall efficacy. The versatility demonstrated in TREM2-independent effects suggests broader applicability, fostering optimism for translational impact.
In conclusion, this breakthrough study not only sheds light on the therapeutic potential of Methoxy-Mycolic Acid in sepsis but also opens up new avenues for the development of targeted therapies. The intricate interplay of molecular, cellular, and epigenetic mechanisms uncovered in this research brings us closer to a more nuanced understanding of immune exhaustion and, consequently, to innovative strategies for restoring immune resilience in the face of sepsis-induced challenges. As the journey from bench to bedside continues, the hope is that M-MA will emerge as a beacon of progress in the battle against sepsis-related complications.
The study findings were published in the peer reviewed journal: iScience.
https://www.sciencedirect.com/science/article/pii/S2589004224001998
For the latest
Medical News, keep on logging to Thailand Medical News.
