BREAKING! Myalgia And Muscle Weakness In Long COVID Is Due To SARS-CoV-2 Downregulating Cellular Muscle Functions, Causing Muscle Injury And Cell Death!
Source: Long COVID - Muscle Weakness And Myalgia Apr 19, 2022 3 years, 1 week, 11 hours, 43 minutes ago
A new study by researchers from State University of Para, Belém-Brazil has found that the conditions of fatigue, muscle weakness, myalgia, and decline in physical and functional performance typically manifested in Long COVID is due to the SARS-CoV-2 virus using the host cellular machinery of the muscle cells for replication, which causes downregulation of cellular muscle functions, resulting in muscle injury and cell death! This is one of the first few studies that demonstrates the involvement of the skeletal muscles in long COVID-19!
To date,
Long COVID involvement of the musculoskeletal system is characterized by the persistence or appearance of symptoms such as fatigue, muscle weakness, myalgia, and decline in physical and functional performance, even at 4 weeks after the onset of acute symptoms of COVID-19.
Muscle injury biomarkers are altered during the acute phase of the disease. The cellular damage and hyperinflammatory state induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may contribute to the persistence of symptoms, hypoxemia, mitochondrial damage, and dysregulation of the renin-angiotensin system.
Furthermore, the occurrence of cerebrovascular diseases, involvement of the peripheral nervous system, and harmful effects of hospitalization, such as the use of drugs, immobility, and weakness acquired in the intensive care unit, all aggravate muscle damage.
The
Long COVID study findings show the involvement of SARS-CoV-2 virus in causing muscle tissue damage and the detailed multifactorial mechanisms of muscle tissue injury.
The study findings were published in the peer reviewed journal: Medical Virology.
https://onlinelibrary.wiley.com/doi/full/10.1002/rmv.2355
The study team elucidated the mechanisms of the impact of long COVID-19 on the musculoskeletal system.
It was found that SARS-CoV-2 infections directly impacts the skeletal muscles or worsens already existing muscle injury, establishing effects on muscles characterized as a) primary, infecting the muscle cells, leading to cellular death; b) secondary, due to derangements in systems such as the respiratory system, central and peripheral nervous systems, c) tertiary, due to COVID-19 cytokine storm (peripheral neuropathy and myopathy); and d) quaternary, due prolonged immobilization (progressive reduction in muscle mass) and hospitalization (mechanical ventilation, drugs, myopathy, and neuropathy).
The SARS‐CoV‐2 coronavirus enters the cells of hosts by binding to angiotensin-converting enzyme 2 (ACE2), and this binding is potentiated by transmembrane serine protease 2 (TMPRSS2).
Importantly, the musculoskeletal tissues express TMPRSS2 and ACE2 and are thus, susceptible to COVID-19. It is highly probable that once bound to ACE2, the virus enters the muscle cells by endocytosis.
It was found that in endosomes, viral proteins fuse to cellular membranes on TMPRSS2 activation, resulting in the release of ribonucleic acid (RNA) into the cytoplasm. The SARS-CoV-2 virus uses the cellular machinery of the muscle cells for replication, which causes downregulation of cellular muscle fun
ctions and thereby causes muscle injury and cell death.
Typically, COVID-19 results in severe hypoxemia and requirements of oxygen supplementation and mechanical ventilation. Diffuse alveolar injury with subsequent aggregation of inflammatory substances, deposition of fibrin and collagen, compromised gaseous exchange, and reduced permeability of alveolar capillaries takes place.
This resulting muscle hypoxia leads to impaired oxidative phosphorylation, decreased protein synthesis, reduced mitochondrial activity, and impaired adenosine triphosphate (ATP) generation with increased protein degradation and the resultant reduction in muscle mass. Hypoxia-inducible factor-1 alpha (HIF‐1α)-mediated increase in the pro-inflammatory cytokine levels takes place.
Anaerobic glycolysis occurs wherein pyruvate is transformed into lactate by the lactate dehydrogenase (LDH) enzyme. Further, activating Notch pathways, inhibiting phosphoinositide 3‐ kinase pathways, and damage to muscle regulatory factors like Myf5, MyoD, MyoD, etc., cause decreased myogenesis.
Furthermore, the reduced peripheral oxygen levels lead to exercise intolerance in long COVID-19.
Further Exacerbation By CNS Injury
Significantly, it was also found that the SARS-CoV-2 invades the Central Nervous System or CNS via the hematogenous route, which involves viral binding to the endothelial cells of the blood‐brain barrier (BBB), an infection of immune-regulatory cells, olfactory neurons, and the liquor route, wherein infected lymphocytes get attached to endothelial cells of the cerebrospinal fluid (CSF) and reach the glial cells and neurons.
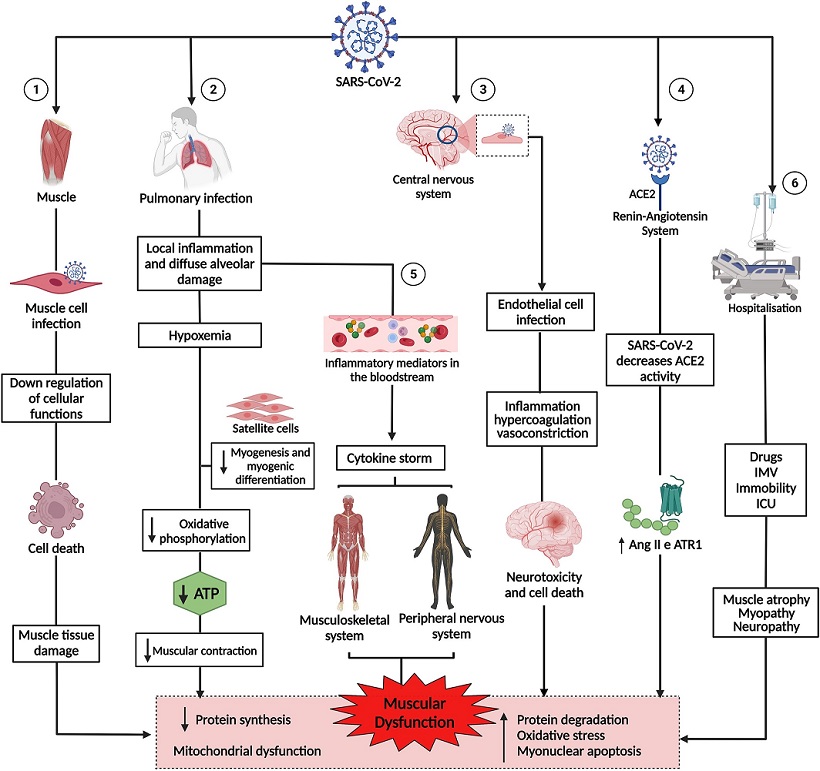 Pathogenesis of muscle dysfunction caused by SARS-CoV-2. 1: SARS-CoV-2 infects the muscle cell & uses cell machinery for replication, resulting in cell death & tissue damage. 2: SARS-CoV-2 infects lung cells, causing a local inflammatory response, diffuse alveolar damage, & hypoxemia, interfering with myogenesis & myogenic differentiation & muscle metabolism & energy production. 3: SARS-CoV-2 infection of endothelial cells results in inflammation, hypercoagulation, & vasoconstriction, leading to neurotoxicity & cell death. 4: The binding of SARS-CoV-2 to angiotensin-converting enzyme 2 (ACE2) negatively regulates the activity of the enzyme, favoring the high expression of angiotensin II (Ang2) & its receptor, angiotensin type 1 receptor (ATR1), leading to muscle atrophy & fibrosis. 5: The exacerbation of inflammation in the lungs increases inflammatory mediators, which are transported by the blood to other organs & systems. In the muscle, inflammatory cytokines increased muscle proteolysis & decreased protein synthesis. In the peripheral nervous system, antibodies attack nerves, causing damage to the axon or myelin. 6: Hospitalization due to COVID-19 can cause muscle damage due to the use of drugs & sedatives, as well as mechanical ventilation & immobility. 1, 2, 3, 4, 5, and 6 lead to muscle dysfunction, characterized by decreased synthesis & increased protein degradation, increased oxidative stress, myonuclear apoptosis, & mitochondrial dysfunction.
Pathogenesis of muscle dysfunction caused by SARS-CoV-2. 1: SARS-CoV-2 infects the muscle cell & uses cell machinery for replication, resulting in cell death & tissue damage. 2: SARS-CoV-2 infects lung cells, causing a local inflammatory response, diffuse alveolar damage, & hypoxemia, interfering with myogenesis & myogenic differentiation & muscle metabolism & energy production. 3: SARS-CoV-2 infection of endothelial cells results in inflammation, hypercoagulation, & vasoconstriction, leading to neurotoxicity & cell death. 4: The binding of SARS-CoV-2 to angiotensin-converting enzyme 2 (ACE2) negatively regulates the activity of the enzyme, favoring the high expression of angiotensin II (Ang2) & its receptor, angiotensin type 1 receptor (ATR1), leading to muscle atrophy & fibrosis. 5: The exacerbation of inflammation in the lungs increases inflammatory mediators, which are transported by the blood to other organs & systems. In the muscle, inflammatory cytokines increased muscle proteolysis & decreased protein synthesis. In the peripheral nervous system, antibodies attack nerves, causing damage to the axon or myelin. 6: Hospitalization due to COVID-19 can cause muscle damage due to the use of drugs & sedatives, as well as mechanical ventilation & immobility. 1, 2, 3, 4, 5, and 6 lead to muscle dysfunction, characterized by decreased synthesis & increased protein degradation, increased oxidative stress, myonuclear apoptosis, & mitochondrial dysfunction.
Numerous past studies have reported an association between COVID‐19 and the cerebrovascular derangements, with hemorrhagic and ischemic stroke, endothelial cell inflammation, and coagulopathy. These effects are associated increase in angiotensin II (Ang II) expression, which causes clot formation, fluid retention, vasoconstriction, and hypertension.
It was also found that COVID-19 associated PNS (Peripheral Nervous System) damage involves neuromuscular junction dysfunctions, myopathies, and polyneuropathies. The hypoxia increases vascular permeability, leading to vasogenic edema. COVID-19, associated with Guillain‐Barré syndrome (GBS), is an example of the PNS involvement of COVID-19, in which flaccid paralysis of lower limb muscles develops rapidly post symptom onset.
Dysregulation Of The Renin-Angiotensin System (RAS), Muscle Hyper-Inflammation.
It was found that the viral entry decreases ACE2 expression, increasing classical RAS activation leading to muscle fibrosis and atrophy and increased production of reactive oxygen species (ROS). The viral immune escape leads to the production of chemokines and cytokines such as interleukins (IL-26,1β,10), tumor necrosis factor‐alpha (TNF‐α), and interferon‐gamma (IFN‐γ), leading to the immune cell recruitment and cytokine storm, that results in muscle damage.
Furthermore, elevated cytokine expression is associated with accelerated loss of muscle tissue, decreased muscle contractility, increased muscle fibrosis, muscle fiber proteolysis, deranged muscle homeostasis, and decreased muscle endurance.
Also, in addition, inhibition of the mammalian target of rapamycin complex 1 (mTORC1) activity leads to dysregulated mitochondrial function and reduced deoxyribonucleic acid (DNA) production.
During hospitalization, prolonged immobilization and prolonged use of glucocorticoids have been reported to result in muscular atrophy, decreased muscle volume, decreased action potentials, decreased density of type II muscle fibers, axonal damage, and decreased insulin‐like growth factor-1 (IGF‐1) levels.
Long COVID Muscle Sequelae
It has been found that Long COVID-19 patients have substantially increased creatine kinase (CK), cholesterol C‐reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, and cortisol, which affect ATP production and cause increased muscle fatigue and thereby decreased ability to perform routine functions.
Importantly, COVID-19 associated sarcopenia causes physical and functional muscular deterioration in muscle volume and strength with decreased satellite cells and activation of anabolic mechanisms.
Also, type 2 diabetes mellitus and obesity exacerbate long COVID-19 complications. Muscle fatigue in long COVID-19 is also associated with cognitive deficits, dyspnea, and reduced lung capacity.
The study review highlights the inflammation, RAS- and hypoxia-associated decrease in muscle mass, strength, size, and volume in long COVID-19, potentiated by prolonged immobilization, drug use, and comorbidities.
The study findings underscore the importance of physical exercise, nutritional monitoring, and other muscle management strategies for long COVID-19 patients.
For the latest research on
Long COVID, keep on logging to Thailand Medical News.

