BREAKING NEWS! COVID-19 Latest: Shocking Discovery That Many COVID-19 Patients In ICU Have Abnormalities Detected On Brain MRI Scans
Source: COVID-19 Latest May 15, 2020 4 years, 11 months, 1 week, 4 days, 12 hours, 48 minutes ago
COVID-19 Latest: Medical researchers from the United States and Turkey have made a shocking discovery that among patients with COVID-19 pneumonia in the intensive care unit (ICU) with neurological symptoms, more than 44 percent of those undergoing magnetic resonance imaging (MRI) have acute findings, according to a research published in the journal
Radiology.
https://pubs.rsna.org/doi/10.1148/radiol.2020201697
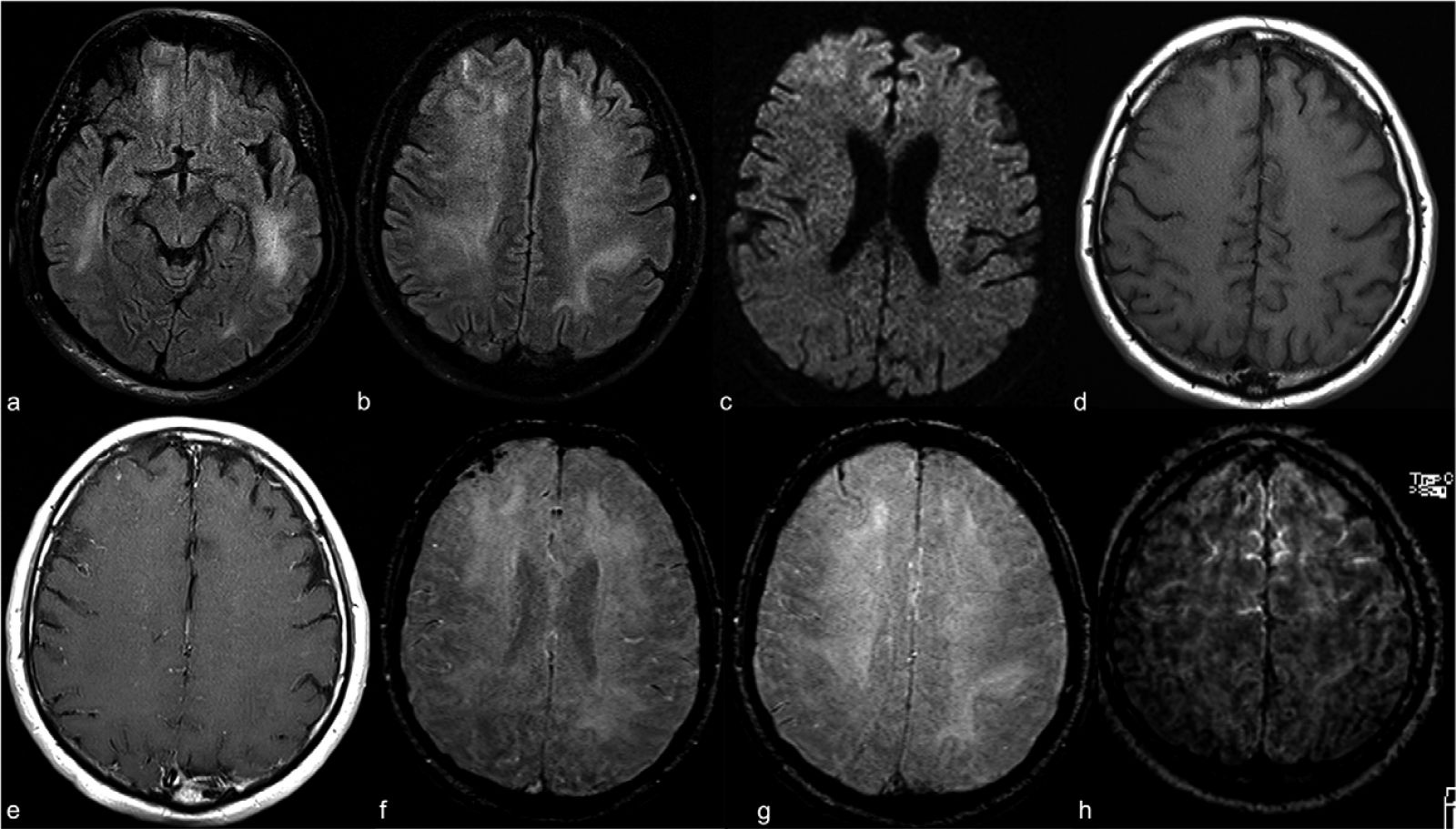 Contrast-enhanced cranial 1.5T MRI examination of a 59-year old intubated male patient with altered mental status despite tapering of sedoanalgesia. Axial FLAIR images at level of midbrain (a) and centrum semiovale (b) demonstrate prominent symmetric white matter hyperintensity and right frontal cortical hyperintensity. There is also prominent linear hyperintensity within frontal sulci. Axial b2000 DWI (c)
Contrast-enhanced cranial 1.5T MRI examination of a 59-year old intubated male patient with altered mental status despite tapering of sedoanalgesia. Axial FLAIR images at level of midbrain (a) and centrum semiovale (b) demonstrate prominent symmetric white matter hyperintensity and right frontal cortical hyperintensity. There is also prominent linear hyperintensity within frontal sulci. Axial b2000 DWI (c)
shows frontal increased signal with corresponding low ADC (images not provided). Axial T1WI (d) shows right frontal sulcal effacement. Post-contrast T1WI (e) shows mild pial-subarachnoid enhancement. Axial SWI at the level of corona radiata (f) and centrum semiovale (g)
demonstrates blooming artifact in the frontal sulci. Post-contrast FLAIR (h) depicts the bilateral leptomeningeal enhancement. ADC apparent diffusion coefficient; FLAIR fluid-attenuated inversion recovery; SWI susceptibility weighted image
The research team led by Dr Sedat G. Kandemirli from the University of Iowa Hospital and Clinics in Iowa City, and colleagues describe brain MRI findings in 235 patients with COVID-19 pneumonia in the ICU who were assessed between March 1 and April 18, 2020, from eight hospitals.
The medical researchers found that 50 of the patients (21 percent) developed neurological symptoms.
Normal brain MRI was performed in 54 percent of the 50 patients with neurologic symptoms.
Of the COVID-19 patients with neurologic symptoms, 44 percent (12 of 27 patients) had acute findings on MRI. Cortical FLAIR signal abnormality was present in 10 of 27 patients (37 percent). In three patients, accompanying subcortical and deep white matter signal abnormality were present on FLAIR images. Cerebrospinal fluid was obtained from five of 10 patients with cortical signal abnormalities; total protein was elevated in four patients. In all five specimens, reverse-transcription polymerase chain reaction assay findings were negative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Accompanying subcortical and deep white matter signal abnormality on FLAIR images were each present in 3 patients. Abnormalities involved the frontal lobe in 4, parietal lobe in 3, occipital lobe in 4, temporal lobe in 1, insular cortex in 3 and cingulate gyrus in 3 patients.
Dr Kandemirli told Thailand Medical News, "This report may help increase awareness for possible neurological involvement of SARS-CoV-2 for patients in the ICU and especially for patients who do not tolerate extubation despite improvement of respiratory findings.”
AN URGENT APPEAL: Please help suppor
t this website by kindly making a donation to sustain this website and also all in all our initiatives to propel further research: https://www.thailandmedical.news/p/sponsorship
Prevailing evidence suggests an association of neurologic manifestations with COVID-19 infection including acute stroke (6%) and altered mental status (15%) (1). Neurotropism of coronavirus may account for the relatively high percentage of neurologic involvement (
6,7).
In addition to neurotropism, another potential mechanism for neurologic manifestations might be related to cytokine storm syndrome (
8). In addition to findings of encephalitis, increased thrombosis rates in coronavirus infection has been reported. In patients with SARS-CoV, increased incidence of deep venous thrombosis and pulmonary embolism was observed despite optimal anticoagulant therapy (
9). Additionally, intracranial arterial stroke cases have been reported in SARS patients receiving IVIG treatment (
9).
A another new study from France reported 84% neurologic signs in 58 COVID-19 patients admitted to ICU. Out of the 13 cranial MRIs performed, leptomeningeal enhancement was noted in 8 cases (
5).. In our studies, the most common imaging finding was cortical signal abnormalities on FLAIR images 10/27 (37%), accompanied by cortical diffusion restriction, leptomeningeal enhancement or cortical blooming artifact in some of these cases.
The significant differential diagnosis for this constellation of findings is infectious or autoimmune encephalitis, seizure, hypoglycemia and hypoxia. (
10-16). The cases with bilateral frontal involvement may have hypoxia as underlying pathogenesis given the underlying respiratory distress and frontotemporal hypoperfusion as demonstrated by Helms et al., in COVID-19 patients admitted to ICU (
5). Cortical microhemorrhages and breakdown of blood-brain barrier can accompany hypoxia which can result in such an imaging pattern. Postictal state is also a plausible etiology for our imaging findings, however the relative symmetry and deep white matter involvement in our cases don’t support postictal changes.
Note that hypoglycemia can act as a potential mimicker, however no episode of hypoglycemia occurred during the ICU course of patients. COVID-19 with its neurotropic potential may result in infectious or autoimmune encephalitis (
3,4). Certain viral and autoimmune encephalitis can have specific pattern of involvement that is helpful to establish a differential list, however nonspecific imaging pattern in our series hinders achieving a specific diagnosis based on MRI (
10).
In addition, the complex clinical course including comorbid conditions like diabetes mellitus, long ICU stay with multidrug regimens, respiratory distress with hypoxia episodes can all act as confounding factors and a clear cause-effect relationship between COVID-19 infection and MRI findings is hard to establish in the absence of more specific CSF findings.
More data is needed to determine which imaging findings are related to neurotropism of COVID-19 and which are related to other etiologies like cytokine storm syndrome, hypoxia, subclinical seizures and critical illness–related encephalopathy.
For more
COVID-19 latest developments, keep logging to Thailand Medical News.
AN URGENT APPEAL: Please help support this website by kindly making a donation to sustain this website and also all in all our initiatives to propel further research: https://www.thailandmedical.news/p/sponsorship
References:
1. Mao L, Wang M, Chen S et al. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a retrospective case series study.
JAMA Neurol 2020. doi: 10.1001/jamaneurol.2020.1127
Google Scholar
2. Desforges M, Le Coupanec A, Stodola JK et al. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis.
Virus Res 2014;194:145-158. doi: 10.1016/j.virusres.2014.09.011
Crossref,
Medline,
Google Scholar
3. Moriguchi T, Harii N, Goto J et al. A first Case of Meningitis/Encephalitis associated with SARS-Coronavirus-2.
Int J Infect Dis 2020. doi: 10.1016/j.ijid.2020.03.062
Crossref,
Medline,
Google Scholar
4. Poyiadji N, Shahin G, Noujaim D et al. COVID-19–associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features.
Radiology 2020:201187. doi: 10.1148/radiol.2020201187
Link,
Google Scholar
5. Helms J, Kremer S, Merdji H et al. Neurologic Features in Severe SARS-CoV-2 Infection.
N Eng J Med 2020. doi: 10.1056/NEJMc2008597
Crossref,
Medline,
Google Scholar
6. Morfopoulou S, Brown JR, Davies EG et al. Human Coronavirus OC43 Associated with Fatal Encephalitis.
N Eng J Med 2016;375(5):497-498. doi: 10.1056/NEJMc1509458
Crossref,
Medline,
Google Scholar
7. Tsai LK, Hsieh ST, Chang YC. Neurological manifestations in severe acute respiratory syndrome.
Acta Neurol Taiwan 2005;14(3):113-119.
Medline,
Google Scholar
8. Mehta P, McAuley DF, Brown M et al. COVID-19: consider cytokine storm syndromes and immunosuppression.
Lancet 2020;395(10229):1033-1034. doi: 10.1016/S0140-6736(20)30628-0
Crossref,
Medline,
Google Scholar
9. Umapathi T, Kor AC, Venketasubramanian N et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS).
J Neurol 2004;251(10):1227-1231. doi: 10.1007/s00415-004-0519-8
Crossref,
Medline,
Google Scholar
10. Koeller KK, Shih RY. Viral and Prion Infections of the Central Nervous System: Radiologic-Pathologic Correlation: From the Radiologic Pathology Archives.
Radiographics 2017;37(1):199-233. doi: 10.1148/rg.2017160149
Link,
Google Scholar
11. Kelley BP, Patel SC, Marin HL et al. Autoimmune Encephalitis: Pathophysiology and Imaging Review of an Overlooked Diagnosis.
AJNR Am J Neuroradiol 2017;38(6):1070-1078. doi: 10.3174/ajnr.A5086
Crossref,
Medline,
Google Scholar
12. Cianfoni A, Caulo M, Cerase A et al. Seizure-induced brain lesions: a wide spectrum of variably reversible MRI abnormalities.
Eur J Radiol 2013;82(11):1964-1972. doi: 10.1016/j.ejrad.2013.05.020
Crossref,
Medline,
Google Scholar
13. Muttikkal TJ, Wintermark M. MRI patterns of global hypoxic-ischemic injury in adults.
J Neuroradiol 2013;40(3):164-171. doi: 10.1016/j.neurad.2012.08.002
Crossref,
Medline,
Google Scholar
14. Bathla G, Policeni B, Agarwal A. Neuroimaging in patients with abnormal blood glucose levels.
AJNR Am J Neuroradiol 2014;35(5):833-840. doi: 10.3174/ajnr.A3486
Crossref,
Medline,
Google Scholar
15. McKinney AM, Sarikaya B, Gustafson C et al. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging.
AJNR Am J Neuroradiol 2012;33(5):896-903. doi: 10.3174/ajnr.A2886
Crossref,
Medline,
Google Scholar
16. Fanou EM, Coutinho JM, Shannon P et al. Critical Illness-Associated Cerebral Microbleeds.
Stroke 2017;48(4):1085-1087. doi: 10.1161/strokeaha.116.016289
Crossref,
Medline,
Google Scholar
