BREAKING NEWS! Florida Study Shows That SARS-CoV-2 Causes Histological Changes To The Gastrointestinal Tract!
Nikhil Prasad Fact checked by:Thailand Medical News Team May 26, 2024 1 year, 6 months, 3 weeks, 8 hours, 44 minutes ago
COVID-19 News: A recent study conducted by a collaborative team from the University of Miami Miller School of Medicine, HCA Florida Kendall Hospital, SASTRA Deemed University, and Molecular Analytics-Miami-USA has revealed significant findings regarding the impact of SARS-CoV-2 on the gastrointestinal tract. While COVID-19 is primarily recognized as a respiratory illness, emerging evidence suggests that the virus can also cause profound changes in the gastrointestinal system. This study covered in this
COVID-19 News report, conducted on murine models, sheds light on the histological changes in the intestines during both acute and long COVID phases, providing valuable insights into the pathogenesis of COVID-induced gastrointestinal complications.
 That SARS-CoV-2 Causes Histological Changes To The Gastrointestinal Tract
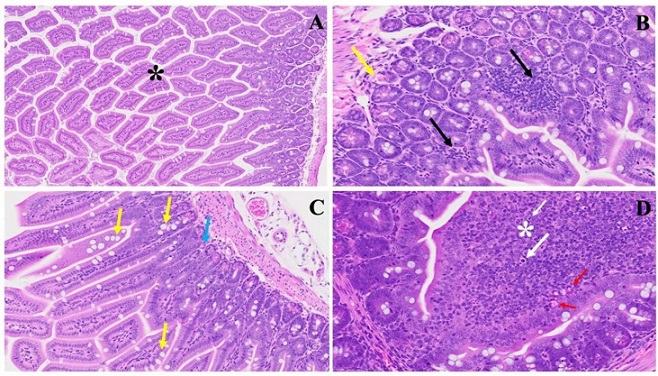
This illustrates the histopathological changes observed in acute extensive intestinal inflammation in the MHV-1 model in small and large intestines. In Panel (A), the typical architecture of the large intestine is depicted, with intact villi highlighted by a star. Panel (B) demonstrates colonic mucosal inflammation and lymphoid hyperplasia, indicated by black arrows, accompanied by microthrombi marked by yellow arrows. In Panel (C), hyperplasia of goblet cells scattered throughout the colonic mucosa is shown (yellow arrows), along with various inflammatory cells within the lamina propria (blue arrow). Panel (D) reveals diffuse proliferation of lymphoid tissue (white star), microbleeding (red arrow), and melanocytosis (white arrows) in the colonic mucosa. These findings provide insight into the pathological features associated with acute significant intestinal changes in the MHV-1 model. (H&E, original magnification 66× (A–D)). MHV-1 infection alone (n = 16), healthy control (n = 7), and SPK-treated mice (n = 5).
That SARS-CoV-2 Causes Histological Changes To The Gastrointestinal Tract
This illustrates the histopathological changes observed in acute extensive intestinal inflammation in the MHV-1 model in small and large intestines. In Panel (A), the typical architecture of the large intestine is depicted, with intact villi highlighted by a star. Panel (B) demonstrates colonic mucosal inflammation and lymphoid hyperplasia, indicated by black arrows, accompanied by microthrombi marked by yellow arrows. In Panel (C), hyperplasia of goblet cells scattered throughout the colonic mucosa is shown (yellow arrows), along with various inflammatory cells within the lamina propria (blue arrow). Panel (D) reveals diffuse proliferation of lymphoid tissue (white star), microbleeding (red arrow), and melanocytosis (white arrows) in the colonic mucosa. These findings provide insight into the pathological features associated with acute significant intestinal changes in the MHV-1 model. (H&E, original magnification 66× (A–D)). MHV-1 infection alone (n = 16), healthy control (n = 7), and SPK-treated mice (n = 5).
The COVID-19 pandemic, which emerged in early 2020, has profoundly impacted global health, resulting in over 7 million deaths worldwide. Beyond the acute phase of the disease, survivors of COVID-19 face numerous long-term health challenges, including cardiovascular abnormalities, liver disturbances, lung complications, kidney issues, and cognitive deficits. The spectrum of long-term complications, often referred to as long COVID, encompasses a wide range of symptoms affecting various bodily systems.
Recent studies have focused on the physiological changes in different organs following prolonged exposure to murine hepatitis virus-1 (MHV-1), a coronavirus used as a model for studying COVID-19 in mice. One significant area of investigation has been the gastrointestinal tract, which has been relatively understudied regarding the long-lasting effects of COVID-19. This research provides essential insights into the histopathological alterations in the small intestine following MHV-1 infection, revealing changes reminiscent of inflammatory bowel disease (IBD) and celiac disease.
Research Objectives and Methodology
The primary aim of this
investigation was to explore the correlation between acute and long-term intestinal modifications in COVID-19. Additionally, the study sought to assess whether the inhibition of viral entry through the utilization of a recently identified synthetic peptide, SPIKENET (SPK), could mitigate or prevent these intestinal alterations. SPK has been shown to effectively impede the binding of the Spike glycoprotein-1 with host receptors and exhibits potent anti-inflammatory properties.
Results - Histopathological Changes in the Gastrointestinal Tract
The study's findings reveal significant histopathological changes in the gastrointestinal tract during both the acute and prolonged phases following MHV-1 infection. Notable alterations include mucosal inflammation, lymphoid hyperplasia, goblet cell hyperplasia, and immune cell infiltration. These changes closely resemble the pathological features observed in IBD. Additionally, MHV-1 infection induces villous atrophy, altered epithelial integrity, and inflammatory responses akin to those seen in celiac disease and IBD.
Acute Phase Changes
During the acute phase of MHV-1 infection, significant intestinal changes were observed. These changes included mucosal inflammation, characterized by the infiltration of various inflammatory cells, including lymphocytes and macrophages. Lymphoid hyperplasia and microthrombus formation were also noted, indicating an active immune response and tissue damage.
Goblet cell hyperplasia, an adaptive response to mucosal injury, was evident in the colonic mucosa. This finding aligns with the increased mucus production often observed in IBD, which serves as a protective mechanism against luminal insults. The presence of inflammatory cells within the lamina propria further suggests an ongoing immune response in the affected tissue.
Prolonged Phase Changes
In the prolonged phase following MHV-1 infection, the study found continued histopathological changes, including villous atrophy, epithelial integrity disruption, and ongoing inflammatory responses. These findings are reminiscent of the chronic inflammation observed in conditions like celiac disease and IBD.
SPK Treatment Efficacy
Encouragingly, the study demonstrated that SPK treatment effectively mitigates the intestinal damage caused by MHV-1 infection. SPK treatment restored tissue architecture, ameliorated inflammatory responses, and normalized goblet cell numbers. These findings suggest the potential of SPK as a therapeutic intervention for COVID-induced gastrointestinal complications.
Long COVID Intestinal Changes
The study also investigated the intestinal changes associated with long COVID. Distinct pathological profiles characterized by inflammatory cell infiltration, altered immune responses, and architectural disruptions were observed. These findings provide valuable insights into the complex nature of long COVID and highlight the potential of SPK to modulate intestinal responses and restore tissue homeostasis.
Discussion
The study's findings underscore the significant impact of viral infections, particularly MHV-1, on the gastrointestinal tract, revealing potential parallels with COVID-induced gastrointestinal complications. The observed histopathological changes, including mucosal inflammation, lymphoid hyperplasia, goblet cell hyperplasia, and immune cell infiltration, closely resemble the pathological features seen in IBD. Additionally, the study highlights the potential of SPK as a therapeutic intervention to mitigate intestinal damage and restore tissue homeostasis.
Implications for Understanding COVID-19 Pathogenesis
Understanding these histopathological alterations provides valuable insights into the pathogenesis of COVID-induced gastrointestinal complications. The findings suggest that SARS-CoV-2 may directly invade enterocytes expressing the angiotensin-converting enzyme 2 (ACE2) receptor, leading to dysregulation of the gut microbiota, immune-mediated damage, and systemic effects of cytokine release. These insights are crucial for developing targeted therapeutic strategies to alleviate gastrointestinal manifestations of viral infections and improve patient outcomes.
Future Directions and Therapeutic Strategies
The study's findings pave the way for future research into the development of targeted therapeutic strategies for COVID-induced gastrointestinal complications. The promising efficacy of SPK as a therapeutic intervention provides hope in addressing the enduring effects of the pandemic. Further research is needed to elucidate the precise mechanisms by which SPK mitigates intestinal damage and to explore its potential applications in other viral-induced gastrointestinal conditions.
Broader Implications for Viral-Induced Intestinal Pathology
The investigation's findings also have broader implications for understanding viral-induced intestinal pathology. The study provides valuable insights into the multifaceted impact of viral infections on the gastrointestinal tract and highlights the importance of studying the long-term effects of viral infections on intestinal health. Understanding these mechanisms is crucial for developing effective therapeutic interventions and improving patient outcomes in the context of viral-induced gastrointestinal complications.
Conclusion
In conclusion, this investigation into the histopathological changes in the small intestine following MHV-1 infection in murine models reveals significant parallels with IBD and celiac disease. The study highlights the profound impact of SARS-CoV-2 on the gastrointestinal tract and underscores the potential of SPK as a therapeutic intervention to mitigate intestinal damage and restore tissue homeostasis. These findings provide valuable insights into the pathogenesis of COVID-induced gastrointestinal complications and offer hope for developing targeted therapeutic strategies to address the enduring effects of the pandemic.
The study findings were published in the peer reviewed journal: Viruses
https://www.mdpi.com/1999-4915/16/6/832
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/post-covid-19-individuals-advised-to-take-probiotics-as-study-shows-sars-cov-2-induced-gut-microbiome-dysbiosis-increases-risk-for-colorectal-cancer
https://www.thailandmedical.news/news/alarming-new-study-data-validates-that-sars-cov-2-spike-proteins-damages-not-just-lung-cells-but-also-intestinal-cells-many-are-dying-slowly-and-silen
https://www.thailandmedical.news/news/breaking-new-international-study-warns-that-sars-cov-2-infections-will-lead-to-cancers-especially-colorectal-cancers-due-to-disruption-in-autophagy
https://www.thailandmedical.news/news/japanese-researchers-warn-that-covid-19-infections-can-cause-gastrointestinal-disorders-that-can-lead-to-deaths
https://www.thailandmedical.news/news/breaking-medical-news-globally-1-in-12-will-develop-gastrointestinal-cancers-and-1-in-16-will-die-from-these-cancers
https://www.thailandmedical.news/news/breaking-news-study-alarmingly-finds-that-sars-cov-2-s-and-n-proteins-drive-invasion-abilities-of-colon-cancer-cells,-resulting-in-rapid-metastasis
