BREAKING News! Researchers Find That COVID-19 Infections And Even COVID-19 Vaccines Can Induce Amyloidosis!
COVID-19 News - COVID-19 Infections - COVID-19 Vaccines - Amyloidosis Jun 25, 2023 1 year, 9 months, 3 weeks, 3 days, 20 hours, 38 minutes ago
Amyloidosis Can Lead To Long Term Organ Dysfunction And Potentially Fatal Outcomes!
COVID-19 News: In a groundbreaking discovery, researchers from esteemed institutions such as Lancashire Teaching Hospitals NHS Foundation Trust-UK, Royal North Shore Hospital & The University of Sydney-Australia, University of Manchester-UK, and Salford Royal Hospital-UK have found a surprising connection between COVID-19 infections, as well as COVID-19 vaccines, and the development of amyloidosis.
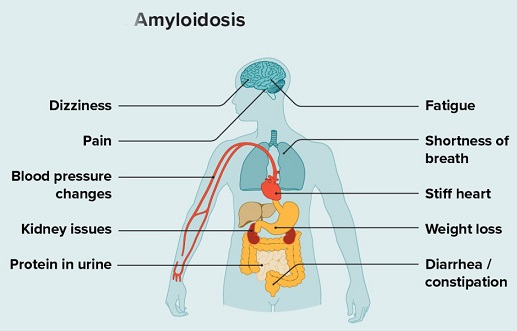
Amyloidosis is a rare disease that occurs when a protein called amyloid builds up in organs. This amyloid buildup can make the organs not work properly. Organs that may be affected include the heart, kidneys, liver, spleen, nervous system and digestive tract. Amyloidosis is a complex disorder characterized by deposited insoluble fibrillar proteins which misfold into β-pleated sheets. The pathogenesis of amyloidosis can vary but can be the result of immune dysregulation that occurs from sustained high inflammatory states, often known as AA amyloidosis.
Amyloidosis is a multifaceted disorder, with various types depending on the protein involved and the organs affected. Systemic amyloidosis, including the commonly observed systemic light-chain (AL) amyloidosis and wide-type transthyretin-associated (ATTRwt) amyloidosis, impacts organs such as the heart, kidneys, liver, lungs, and gastrointestinal and nervous systems. Another type, AA amyloidosis, occurs as a result of abnormal aggregations of serum amyloid A protein due to prolonged infection, chronic illness, or high inflammatory states. The similarities between the health complications associated with COVID-19 and amyloidosis have prompted researchers to explore the potential connections between the two conditions.
Initially regarded as a respiratory disease, COVID-19 has revealed its multifaceted nature with a range of extra-pulmonary manifestations similar to those observed in amyloidosis patients. Shared features include oxidative stress, chronic inflammation, and thrombotic risks. Viral infections have been known to trigger autoimmune conditions, and amyloidosis appears to be no exception. Reports have emerged in recent months documenting new-onset and relapsed amyloidosis cases following COVID-19 infection and vaccination.
https://www.sciencedirect.com/science/article/pii/S2214442021000826
https://www.tandfonline.com/doi/full/10.1080/19336896.2023.2201151
https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.120.050754
https://link.springer.com/article/10.1007/s00392-021-01910-2
https://onlinelibrary.wiley.com/doi/full/10.1111/his.14134
e/pii/S1198743X22001604">https://www.sciencedirect.com/science/article/pii/S1198743X22001604
https://www.sciencedirect.com/science/article/pii/S1053249823004916
https://www.sciencedirect.com/science/article/pii/S1201971221003647
https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-2083732
Thailand Medical News in its previous
COVID-19 News reports did cover about the discovery of Serum Amyloid A (SAA) in COVID-19 patients.
https://www.thailandmedical.news/news/coronavirus-news-study-finds-that-serum-amyloid-a-saa-linked-to-covid-19-inflammation-and-disease-severity-
https://www.thailandmedical.news/news/breaking-university-of-oklahoma-study-indicates-that-sars-cov-2-might-cause-serum-amyloid-a-saa-amyloidosis-and-even-alzheimer-ultimately
However, the precise pathophysiological associations between COVID-19 and amyloidosis still remain unclear.
The findings of this new research are significant and worrisome, shedding light on the complex interplay between COVID-19 and amyloidosis and uncovering potential pathways for future investigations including those involved with Long COVID.
To shed light on this intriguing relationship, the study team conducted a scoping review based on a systematic search of available evidence. The review aimed to evaluate current perspectives and identify knowledge gaps that require further exploration. One significant finding was the potential role of the serum amyloid A (SAA) protein in linking COVID-19 severity and amyloidosis. SAA, a marker of acute phase response, exhibits high levels during systemic infection, inflammation, and cytokine storms. Elevated SAA concentrations have been associated with worse COVID-19 infection outcomes, including increased mortality and acute respiratory distress syndrome (ARDS). However, the long-term risk of developing amyloidosis for individuals with prolonged COVID-19 infection remains unknown.
The proposed pathophysiological associations of COVID-19 infection-induced amyloidosis suggest that the severity of the disease generates increased protein aggregation and amyloid formation. COVID-19 infection triggers a cascade of events, including the release of chemokines, activation of neutrophils, upregulation of cytokines and proteases, loss of endothelial cell permeability, and subsequent systemic inflammation and injury. The production of abnormal proteins, such as pulmonary surfactant protein SP-C, may result from these processes.
Additionally, oxidative stress induced by COVID-19 infection can stimulate the misfolding and aggregation of metastable proteins, contributing to amyloid formation.
Similarly, the pathophysiological associations of COVID-19 vaccination-induced amyloidosis are still being investigated. Limited reported cases have made it challenging to understand the mechanisms behind this phenomenon. However, raised SAA levels have been observed in COVID-19 vaccine-induced diseases, such as IgA vasculitis and nephropathy, and other autoimmune conditions. The shared symptoms between viral-induced and vaccine-induced amyloidosis are of particular interest to researchers. Some individuals who have received COVID-19 vaccines have reported symptoms similar to those seen in amyloidosis, including persistent fatigue, joint pain, and organ dysfunction. However, it is important to note that these cases are extremely rare and have not been conclusively linked to the vaccines.
The complex relationship between COVID-19, vaccines, and amyloidosis requires further investigation to understand the underlying mechanisms. It is crucial to differentiate between coincidental occurrences and causal relationships. Large-scale studies and long-term follow-ups are needed to determine the true risk and prevalence of amyloidosis following COVID-19 infection or vaccination.
The implications of these findings extend beyond COVID-19 and amyloidosis. Understanding the interplay between viral infections, vaccines, and the development of rare diseases could provide valuable insights for future research and public health strategies. It underscores the need for ongoing monitoring, surveillance, and evaluation of potential adverse effects associated with viral infections and vaccinations.
While the news of a potential link between COVID-19, vaccines, and amyloidosis is noteworthy, it is essential to approach these findings with caution. Research in this field is still in its early stages, and more evidence is needed to establish definitive conclusions.
The study findings were published in the peer reviewed journal: Vaccines.
https://www.mdpi.com/2076-393X/11/7/1139#B64-vaccines-11-01139
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
