BREAKING! Not The Type Of ADE Expected! Opsonization By Non-Neutralizing Antibodies Confer Protection To SARS-CoV-2 By Reducing Phagocytosis!
Source: ADE-SARS-CoV-2 Oct 27, 2021 3 years, 5 months, 4 weeks, 2 days, 2 hours, 30 minutes ago
The alarming study findings by researchers from the Faculty of Medicine, Lund University-Sweden, Public Health Agency of Sweden, Adlego Biomedical AB-Sweden, Tanea Medical Ab-Sweden, jSciEd Solutions-Sweden, University of Zurich-Switzerland and Ecole Normale Superieure Paris-Saclay-France indicates that the normal type of ADE or Antibody-enhancement phenomena that most were expecting to be manifested by the SARS-CoV-2 instead materializes in a different form ie the opsonization by non-neutralizing antibodies confer protection to SARS-CoV-2 by reducing phagocytosis!
.jpg)
Opsonization is an immune process which uses opsonins to tag foreign pathogens for elimination by phagocytes. Without an opsonin, such as an antibody, the negatively-charged cell walls of the pathogen and phagocyte repel each other.
Spike-specific antibodies are central to effective COVID19 immunity. Research efforts have focused on antibodies that neutralize the ACE2-Spike interaction but not on non-neutralizing antibodies. Antibody-dependent phagocytosis is an immune mechanism enhanced by opsonization, where typically, more bound antibodies trigger a stronger phagocyte response.
The study findings show that Spike-specific antibodies, dependent on concentration, can either enhance or reduce Spike-bead phagocytosis by monocytes independently of the antibody neutralization potential.
Shockingly it was found that both convalescent patient plasma and patient-derived monoclonal antibodies lead to maximum opsonization already at low levels of bound antibodies and is reduced as antibody binding to Spike protein increases.
Furthermore, the study finding show that this Spike-dependent modulation of opsonization seems to affect the outcome in an experimental SARS-CoV-2 infection model. These results suggest that the levels of anti-Spike antibodies could influence monocyte-mediated immune functions and propose that non-neutralizing antibodies could confer protection to SARS-CoV-2 infection by mediating phago-cytosis.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2021.10.14.464464v1
The COVID-19 disease, which is caused by the SARS-CoV-2 coronavirus, has caused almost 5 million deaths worldwide, as well as a significant impact on societal health throughout the world.
In the early stages of the COVID-19 pandemic, both monoclonal antibodies and convalescence plasma were used to treat patients.
Basically, two monoclonal antibody cocktails, of which included both casirivimab and imdevim, as well as bamlanivimab and etesevim, were found to target the SARS-CoV-2 spike protein. As a result of their success during Phase III clinical trials, these two antibody treatments quickly received emergency use authorization (EUA) by the United States Food and Drug Administration (FDA).
These trials indicated that the use of these antibody cocktails led to a reduction in symptoms, hospitalization, and mortality that was associated with early-stage COVID-19.
However, the antibiotic cocktails did not show any benefit in case of severe COVID-19 infections.
These aforementioned therapeutic antibodi
es have been categorized as neutralizing antibodies. Neutralizing antibodies comprise only a fraction of the total antibodies that are generated by B-cells against the SARS-CoV-2 spike protein.
Importantly, non-neutralizing antibodies comprise a majority of the humoral response against a pathogen. These antibodies are also known to have other immunological functions such as complement-dependent immune activation and viral phagocytosis. By carrying out either virus or cellular phagocytosis, phagocytic cells help to reduce the viral load.
It was found that in the case of certain viral infections such as Respiratory Syncytial Virus (RSV), SARS-CoV-2, and Dengue, the level of neutralizing antibodies is found to be insufficient. This allows non-neutralizing antibodies to mediate entry of the virions into the host immune cells resulting in an Antibody-Dependent-Enhancement (ADE). This further results in increased infection, along with worsened patient outcomes. To date, no study has shown evidence of ADE in the case of COVID-19 infections.
.jpg) Convalescent patient plasma reduces Spike-monocyte interaction. a Biotinylated Spike protein was conjugated to fluorescent (APC) streptavidin microspheres and was opsonized with three convalescent patient plasma concentrations (1%, 0.1%, and 0.01%). The beads were then mixed with THP-1 cells at a ratio of 2:1, and the association was measured using flow cytometry. Cells that had signal in the APC channel were considered positive. The gating strategy is shown in the top right. b The same samples of THP-1 cells and beads from (a) were fixed with methanol and stained with a fluorescent (FITC) Fab anti-human Fab secondary antibody. The samples were analyzed for human antibody (opsonin) binding to the Spike-beads using flow cytometry. The gating strategy is shown in the top right. The data presented are from three independent experiments. Error bars represent the SD. Statistical significance was assessed using two-way ANOVA with Dunnett’s multiple comparison correction. * denotes p 0.05, ** for p 0.01, *** for p 0.001 and **** for p 0.0001).
The study findings showed evidence that convalescent patient plasma and monoclonal anti-spike antibodies were able to induce phagocytosis at high antibody concentrations. This study also demonstrated that neutralization potential did not have any effect on the activation and inhibition of phagocytosis. This study also utilized an experimental animal infection model to determine the role of non-neutralizing antibodies in providing protection against SARS-CoV-2.
Convalescent patient plasma reduces Spike-monocyte interaction. a Biotinylated Spike protein was conjugated to fluorescent (APC) streptavidin microspheres and was opsonized with three convalescent patient plasma concentrations (1%, 0.1%, and 0.01%). The beads were then mixed with THP-1 cells at a ratio of 2:1, and the association was measured using flow cytometry. Cells that had signal in the APC channel were considered positive. The gating strategy is shown in the top right. b The same samples of THP-1 cells and beads from (a) were fixed with methanol and stained with a fluorescent (FITC) Fab anti-human Fab secondary antibody. The samples were analyzed for human antibody (opsonin) binding to the Spike-beads using flow cytometry. The gating strategy is shown in the top right. The data presented are from three independent experiments. Error bars represent the SD. Statistical significance was assessed using two-way ANOVA with Dunnett’s multiple comparison correction. * denotes p 0.05, ** for p 0.01, *** for p 0.001 and **** for p 0.0001).
The study findings showed evidence that convalescent patient plasma and monoclonal anti-spike antibodies were able to induce phagocytosis at high antibody concentrations. This study also demonstrated that neutralization potential did not have any effect on the activation and inhibition of phagocytosis. This study also utilized an experimental animal infection model to determine the role of non-neutralizing antibodies in providing protection against SARS-CoV-2.
The
ADE-SARS-CoV-2 research began with in vitro transfection of HEK293 cells with plasmids for expression of the spike protein, as well as antibodies. SARS-CoV-2 anti-spike antibodies were selected with the help of phage display technology.
Blood specimens were also collected from 20 patients with mild, moderate, or severe COVID-19. These blood samples were used for the isolation of B-cells. Spike proteins were incubated with the B-cells followed by bulk cell sorting, genome sequencing, and antibody reactivity screening.
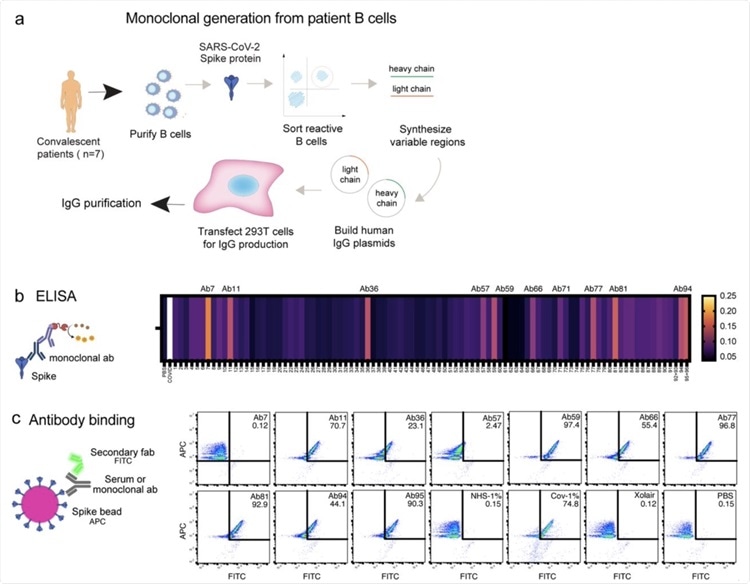 Generation of Spike-reactive human monoclonal antibodies. a) Human monoclonal antibodies were generated from convalescent donor B cells through single-cell sequencing technology. 96 antibodies derived from Spike-reactive human B cells were produced in HEK293F cells. b) Cell culture supernatants containing the antibodies were assayed by ELISA for reactivity against immobilized Spike protein. Serum from a COVID19 patient was used as a positive control. The data represent three replicate ELISAs where reproducibly reactive antibodies are indicated with their names above the heatmap. c) Antibodies which were Spike-reactive in (b) were assayed for reactivity to Spike immobilized on beads. Fluorescent (APC) Streptavidin beads coated with biotinylated Spike protein were incubated with HEK293F-produced antibodies at a concentration of 1 µg/ml. The beads were then stained with a fluorescent (FITC) secondary anti-Fab antibody. The beads were analyzed by flow cytometry. Antibodies that shifted the beads into the FITC-positive gate were deemed reactive.
Generation of Spike-reactive human monoclonal antibodies. a) Human monoclonal antibodies were generated from convalescent donor B cells through single-cell sequencing technology. 96 antibodies derived from Spike-reactive human B cells were produced in HEK293F cells. b) Cell culture supernatants containing the antibodies were assayed by ELISA for reactivity against immobilized Spike protein. Serum from a COVID19 patient was used as a positive control. The data represent three replicate ELISAs where reproducibly reactive antibodies are indicated with their names above the heatmap. c) Antibodies which were Spike-reactive in (b) were assayed for reactivity to Spike immobilized on beads. Fluorescent (APC) Streptavidin beads coated with biotinylated Spike protein were incubated with HEK293F-produced antibodies at a concentration of 1 µg/ml. The beads were then stained with a fluorescent (FITC) secondary anti-Fab antibody. The beads were analyzed by flow cytometry. Antibodies that shifted the beads into the FITC-positive gate were deemed reactive.
Subsequent to this, three different assays were carried out including spike-THP-1 association assays, Pseudotyped virus neutralization assays, and Bead-based neutralization assay. Immunoglobulin G (IgG)-antigen interaction was determined followed by determination of epitope location by enzyme-linked immunosorbent assay (ELISA).
Also detailed surface plasmon resonance studies were carried out for the assessment of IgG specificity. Finally, antibodies were cross-linked to the spike proteins followed by peptide analysis using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
The further in vivo experiments involved the use of transgenic mice that carried the humane angiotensin-converting enzyme 2 (ACE2) gene and were inoculated with SARS-CoV-2. The mice were divided into six groups, with seven mice in each group. Both neutralizing and non-neutralizing antibodies were administered intraperitoneally in one single dose. The infection was allowed to proceed for seven days, following which the mice were euthanized.
The study findings indicated that at a high plasma concentration, there was a reduction in the binding of TPH1 monocytes and the spike protein. This reduction was found to be independent of the age, gender, and disease severity of the patient. Experiments with monoclonal antibodies detected ten spike-reactive antibodies, out of which nine were found to bind to spike beads as detected by flow cytometry.
Interestingly the results of the epitope analysis indicated that antibodies could bind the spike protein but they did not compete with the binding site of human ACE2. These antibodies included Ab11, 57, 59, 66, 77, 81, 94.
The antibody Ab94 could bind to both the receptor-binding domain (RBD) and N-terminal domain (NTD). Ab59 was a neutralizing antibody, while the others were non-neutralizing antibodies. Overall, Ab59 was found to be most effective in SARS-CoV-2 neutralization.
The study findings also indicated that the spike-specific monoclonal antibodies that were obtained from COVID-19 patients had a dose-dependent effect on the spike-THP-1 cell interactions. This effect was independent of the spike-ACE2 neutralization capability of the monoclonal antibodies.
While the study findings indicated that the monoclonal antibodies were effective in the mediation of phagocytosis, a threshold that led to a reduction in interaction efficiency was identified.
The detailed determination of the antibodies’ function in a physiological context took place with the help of mice models. The results from the in vivo study indicated that too high doses of neutralizing antibodies were not beneficial for treatment.
Surprisingly, non-neutralizing antibodies were also found to provide protection against SARS-CoV-2.
Hence the study findings show a concentration-dependent modulation of phagocytosis that was brought about by anti-spike antibodies. It may also help to indicate the unclear clinical benefit of COVID-19 treatment with monoclonal antibodies.
The study team added, “It is widely accepted that a strong positive correlation exists between COVID-19 disease severity and antibody titers. High antibody titers are generally associated with severe disease and hospitalization. The high titers are thought to be a consequence of the severe infection. Our results pose a new question: could the high anti-Spike titers seen in hospitalized patients instead (at least partially) contribute to the immune dysregulation and worsening patient outcomes? These questions are relevant in the light of the U.S.FDA’s recommendation not to use monoclonal antibodies in hospitalized COVID-19 patients (and who are seropositive) due to possible worsening of symptoms. At the same time, it is important to note that convalescent plasma treatment for COVID-19 was shown to be neither beneficial nor detrimental.
https://www.thelancet.com/article/S0140-6736(21)00897-7/fulltext Overall, the results presented in this study highlight a concentration-dependent modulation of phagocytosis by anti-Spike antibodies. This modulation phenomenon might help explain the unclear clinical benefit seen with monoclonal antibody treatment for COVID19. This modulation is seen in patient material and translates well to animal infection experiments. This opens several avenues for further experimentation. The biophysical mechanism underlying the antibody-mediated phagocytic modulation is an exciting topic to pursue, as are the bridging immune steps between phagocytosis and animal protection.”
For the latest
ADE-SARS-CoV-2 research, keep on logging to Thailand Medical
.jpg)
.jpg) Convalescent patient plasma reduces Spike-monocyte interaction. a Biotinylated Spike protein was conjugated to fluorescent (APC) streptavidin microspheres and was opsonized with three convalescent patient plasma concentrations (1%, 0.1%, and 0.01%). The beads were then mixed with THP-1 cells at a ratio of 2:1, and the association was measured using flow cytometry. Cells that had signal in the APC channel were considered positive. The gating strategy is shown in the top right. b The same samples of THP-1 cells and beads from (a) were fixed with methanol and stained with a fluorescent (FITC) Fab anti-human Fab secondary antibody. The samples were analyzed for human antibody (opsonin) binding to the Spike-beads using flow cytometry. The gating strategy is shown in the top right. The data presented are from three independent experiments. Error bars represent the SD. Statistical significance was assessed using two-way ANOVA with Dunnett’s multiple comparison correction. * denotes p 0.05, ** for p 0.01, *** for p 0.001 and **** for p 0.0001).
Convalescent patient plasma reduces Spike-monocyte interaction. a Biotinylated Spike protein was conjugated to fluorescent (APC) streptavidin microspheres and was opsonized with three convalescent patient plasma concentrations (1%, 0.1%, and 0.01%). The beads were then mixed with THP-1 cells at a ratio of 2:1, and the association was measured using flow cytometry. Cells that had signal in the APC channel were considered positive. The gating strategy is shown in the top right. b The same samples of THP-1 cells and beads from (a) were fixed with methanol and stained with a fluorescent (FITC) Fab anti-human Fab secondary antibody. The samples were analyzed for human antibody (opsonin) binding to the Spike-beads using flow cytometry. The gating strategy is shown in the top right. The data presented are from three independent experiments. Error bars represent the SD. Statistical significance was assessed using two-way ANOVA with Dunnett’s multiple comparison correction. * denotes p 0.05, ** for p 0.01, *** for p 0.001 and **** for p 0.0001).