BREAKING! Research News: Oxford Study Discovers That SARS-CoV-2 Evades Host Immune Responses By Proteolytic Cleavage Of Nucleocapsid
Source: Research News Oct 08, 2020 4 years, 6 months, 2 weeks, 4 days, 17 hours, 14 minutes ago
Research News: Scientist from the Physical and Theoretical Chemistry Laboratory at the University of Oxford have made a startling discovery as to yet another one of the many modes that the SARS-CoV-2 coronavirus is able to evade human host immune responses once it is inside the host cells and replicating.
The study team discovered that that the nucleocapsid protein of SARS-CoV-2 coronavirus undergoes autoproteolysis, and that the resulting products can escape the host immune responses and facilitate viral proliferation.
The research findings are published on a preprint server and have yet to be peer reviewed.
https://www.biorxiv.org/content/10.1101/2020.10.06.328112v1
or
https://www.biorxiv.org/content/10.1101/2020.10.06.328112v1.full.pdf
Studies have shown that the genome of SARS-CoV-2 encodes 25 non-structural proteins and 4 structural proteins, including the spike, membrane, nucleocapsid, and envelop proteins. Besides spike protein, the nucleocapsid is the most abundant protein that encapsulates the viral RNA.
The SARS-CoV-2 nucleocapsid (N) protein is the most immunogenic of the structural proteins and plays essential roles in several stages of the virus lifecycle including viral replication/proliferation, virion assembly, host cell cycle regulation, and host immune system evasion.
The nucleocapsid (N) protein is comprised of two major structural domains: the RNA binding domain, which interacts with viral and host RNA, and the oligomerization domain which assembles to form the viral core.
Interestingly, studies have found that host adaptive immune responses to nucleocapsid is more sensitive than the spike protein, which makes nucleocapsid an important therapeutic target.
In the context of many other viruses, many in vitro studies have shown that molecules targeting nucleocapsid proteins are effective in inhibiting viral replication, transcription, and virion assembly.
Considering the critical significance of nucleocapsid protein in anti-viral drug development, the study team aimed at deciphering the involvement of nucleocapsid protein in the SARS-CoV-2 lifecycle.
It must be noted that there are two major structural domains of the nucleocapsid protein: the RNA binding domain and the oligomerization domain, separated by an extended, flexible linker region.
Importantly, the RNA binding domain interacts with both host and viral RNA, whereas the oligomerization domain forms the viral core.
The study team utilizing native mass spectrometry, observed and found that that the dimers of full-length nucleocapsid protein interact with RNA, indicating that dimers, and not monomers, are the functional unit of ribonucleoprotein assembly.
Significantly the team observed that nucleocapsid protein dimers preferentially bind RNA through the GGG motif, which is known to form stem-loop structures.
Surprisingly, the researchers observed that autoproteolytic events occur within the linker region of full-length nucleocapsid protein, ge
nerating at least five proteoforms.
In order to identify proteolytic cleavage sites, the study team next sequenced these proteoforms using electron transfer dissociation and higher-energy collision-induced dissociation and validated the sequences by peptide mapping. The team observed that proteolytic cleavage sites are highly conserved in the SARS-CoV-2 genome. In general, the cleavage sites were found to be immediately next to a hydrophobic residue.
It was found that of the five proteoforms of the nucleocapsid protein (N1-209, N1-220, N1-273, N156-419, and N1-392), N1-209 and N1-220 mainly contain the RNA binding domain; N1-273 contains a significant part of the RNA binding domain, followed by the linker region and a small part of the oligomerization domain; and N156-419 primarily contains the oligomerization domain and a small part of the RNA binding domain.
Interestingly with regards to the functional properties of these 5 proteforms, the researchers observed that unlike the full-length nucleocapsid protein, the N-terminal proteoforms preferentially interact with RNA via the GGG motif and do not undergo pH-dependent alteration in the oligomeric state.
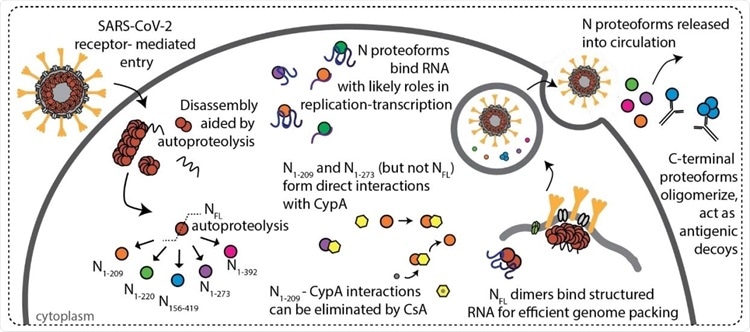 Scheme depicting features of SARS-CoV-2 N protein during infection. N protein undergoes autoproteolysis in a highly specific fashion to produce N1-209, N1-220, N1-273, N156-419, and N1-392. NFL and N proteoforms bind RNA with a preference for structured RNA and NFL dimers are likely functional units of assembly in ribonucleoprotein complexes. Immunophilin CypA binds directly to N1-209 and N1-273, but not NFL or N1-220, and the interaction can be inhibited through addition of cyclosporin A (CsA). N156-419 is readily detectable by antibodies, suggesting a role in immune evasion via the formation of antigenic decoys.
Scheme depicting features of SARS-CoV-2 N protein during infection. N protein undergoes autoproteolysis in a highly specific fashion to produce N1-209, N1-220, N1-273, N156-419, and N1-392. NFL and N proteoforms bind RNA with a preference for structured RNA and NFL dimers are likely functional units of assembly in ribonucleoprotein complexes. Immunophilin CypA binds directly to N1-209 and N1-273, but not NFL or N1-220, and the interaction can be inhibited through addition of cyclosporin A (CsA). N156-419 is readily detectable by antibodies, suggesting a role in immune evasion via the formation of antigenic decoys.
In contrast however, the oligomeric state of the C-terminal proteoforms was found to be sensitive to both high and low pH conditions.
The study team mass spectrometry, observed that although full-length nucleocapsid protein binds to an anti-nucleocapsid monoclonal antibody, proteoforms lack C-terminal residues and do not interact with the antibody.
Hence this indicates that the antigenic region of nucleocapsid protein is located strategically toward the C-terminus.
With regards to the interaction between proteoforms and vital replication components of SARS-CoV-2, such as cyclophilin A, the study team observed N-terminal proteoforms (N1-209 and N1-273) directly interact with cyclophilin A, and this interaction can be inhibited by administering immunosuppressants.
Note that the direct binding of N1-209 and N1-273 to cyclophilin A, can be inhibited by the approved immunosuppressant drug cyclosporine.
The key implications and findings of this study is that N-terminal proteolytic products generated by autoproteolysis of SARS-CoV-2 nucleocapsid protein can evade the antibody-mediated immune responses by removing the C-terminal residues. Also, these N-terminal proteolytic products can facilitate viral proliferation by directly binding vital components of the viral replication process.
The research findings also indicate that inhibition of the proteolytic process can suppress the immune evasion and proliferation of SARS-CoV-2, hence can approached as a new way of therapeutic intervention.
The study team said, “The results presented here do prompt further investigation of a number of therapeutic strategies including inhibition of the autoproteolyis reaction, perturbation of N-protein RNA binding, and prevention of the cyclophilin A proteoform interactions. Given the likelihood that no one intervention is likely to ameliorate the complex symptoms of COVID-19 infection, our findings contribute proteoform-specific information that may guide some of the many therapies currently under investigation.”
For the latest
Research News, keep on logging to Thailand Medical News.

