BREAKING! Researchers Discover That SARS-CoV-2 ORF10 Proteins Destroys And Causes Loss Of Motile Cilia Found On Host Epithelial Cells!
Source: Medical News - SARS-CoV-2 ORF10 And Cilia Loss Jun 16, 2022 3 years, 6 months, 4 days, 23 hours, 10 minutes ago
A study led by researchers from the Chinese Academy of Sciences-Beijing that also involved other research hospitals and universities in China has alarmingly found that the SARS-CoV-2 ORF10 proteins are able to cause the degradation of ciliary proteins and cause the loss of motile cilia on the host epithelial cells.
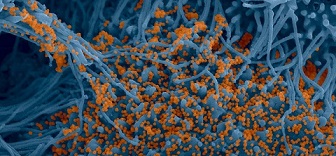
The study findings demonstrate that the viral protein ORF10 induces the degradation of an array of ciliary proteins, leading to the disappearance of a whole organelle involved in viral particle clearance.
But at the same time, Thailand
Medical News would like to add that cilia damage and loss in various other epithelial cells can also lead to a whole list of health issues and also long-term medical complications including some we are now witnessing in Long COVID patients.
Cilia, which are tiny hair-like structures that play important functions involving locomotion and sensory functions. They are primarily responsible for locomotion, either of the cell itself or of fluids on the cell surface. They are also involved in mechanoreception.
Implications Of Cilia Damage or Loss:
Ciliary defects and cilia loss can lead to a number of human diseases. Loss and defects in cilia adversely affect many critical signaling pathways essential to embryonic development and to adult physiology, and thus offer a plausible hypothesis for the often multi-symptom nature of diverse ciliopathies. Known ciliopathies include primary ciliary dyskinesia(PCD), Bardet–Biedl syndrome, polycystic kidney and liver disease, nephronophthisis, Alström syndrome, Meckel–Gruber syndrome, Sensenbrenner syndrome,some forms of retinal degeneration, nephronophthisis, Senior–Løken syndrome
Also defects of the primary cilium in the renal tubule cells can lead to polycystic kidney disease (PKD). Defects in cilia cells are linked to obesity and often pronounced in type 2 diabetes. Several studies already showed impaired glucose tolerance and reduction in the insulin secretion in the ciliopathy models.
Epithelial sodium channels (ENaCs) that are expressed along the length of cilia regulate periciliary fluid level. Decreased activity of ENaCs due to loss or damage of cilia result in multisystem pseudohypoaldosteronism, that is associated with fertility problems. In cystic fibrosis that results from mutations in the chloride channel CFTR, ENaC activity is enhanced leading to a severe reduction of the fluid level that causes complications and infections in the respiratory airways. There is an association of primary ciliary dyskinesia with left-right anatomic abnormalities such as situs inversus (a combination of findings is known as Kartagener syndrome), and situs ambiguus (also known as Heterotaxy syndrome).These left-right anatomic abnormalities can also result in congenital heart disease.
SARS-CoV-2 And Cilia Damage And Loss.
The SARS-CoV-2 coronavirus is the causal pathogen of the ongoing global COVID-19 pandemic.
Loss of smell and taste are symptoms of COVID-19, and may be related to cilia dysfunction.
Th study team found that the SARS-CoV-2 ORF10 increases the overall E3 ligase activity of the CUL2ZYG11B complex
by interacting with ZYG11B. Enhanced CUL2ZYG11B activity by ORF10 causes increased ubiquitination and subsequent proteasome-mediated degradation of an intraflagellar transport (IFT) complex B protein, IFT46, thereby impairing both cilia biogenesis and maintenance.
The study findings also showed that exposure of the respiratory tract of hACE2 mice to SARS-CoV-2 or SARS-CoV-2 ORF10 alone results in cilia-dysfunction-related phenotypes, and the ORF10 expression in primary human nasal epithelial cells (HNECs) also caused a rapid loss of the ciliary layer.
The study findings demonstrate how SARS-CoV-2 ORF10 hijacks CUL2ZYG11B to eliminate IFT46 and leads to cilia dysfunction, thereby offering a powerful etiopathological explanation for how SARS-CoV-2 causes multiple cilia-dysfunction-related symptoms specific to COVID-19.
The study findings were published in the peer reviewed Journal of Cell Biology.
https://rupress.org/jcb/article-abstract/221/7/e202108015/213272/SARS-CoV-2-ORF10-impairs-cilia-by-enhancing?redirectedFrom=fulltext
Typically, the motile cilia that line the epithelial tissue of the airways are considered the first line of defense against pathogens that cause several respiratory diseases.
It is known that the airway epithelium consists of one to two hundred cilia at their surface that primarily secrete viscoelastic mucus, which helps to trap inhaled particles, including viruses.
The various particles that are trapped within mucus are then brought towards the pharynx through the coordinated beating of cilia, which subsequently allows for the mucus to be swallowed or expelled.
Studies have shown that several respiratory pathogens have developed different mechanisms to escape the mucociliary clearance pathway.
The SARS-CoV-2 coronavirus is also known to directly infect ciliated cells and destroy cilia.
The study team identified the SARS-CoV-2 open reading frame 10 (ORF10) protein to be responsible for the loss of cilia as a result of its role in promoting the ubiquitin-dependent degradation of ciliary proteins.
The SARS-CoV-2 ORF10 appears to interact with the E3 ubiquitin ligase adapter ZYG11B. E3 ubiquitin ligases also interact with E2 ubiquitin-conjugating enzymes and E1 ubiquitin-activating enzymes to attach to ubiquitin and target proteins. Notably, many viruses have evolved mechanisms to escape the ubiquitin/proteasome pathway by encoding E3 mimic proteins that aid in the degradation of antiviral cellular proteins.
Another research team from Institut Pasteur-France delved deeper into the Chinese study to determine whether the interaction between ORF10 and ZYG11B promotes the dissemination of SARS-CoV-2.
The initial Chinese study involved pull-down experiments to confirm the interaction between the SARS-CoV-2 ORF10 and ZYG11B. Tandem Mass Tag (TMT) proteomics analysis was used to determine the effect of ORF10 on the cellular proteome, followed by Western blot analysis. The dose-dependency of the ORF10 effect was also evaluated.
Vivo studies involving human angiotensin-converting enzyme 2 (ACE2) knock-in mice were conducted using a lentiviral vector carrying ORF10 to better understand the role of this viral protein during SARS-CoV-2 infection.
Interestingly, the in vitro interaction between the SARS-CoV-2 ORF10 and ZYG11B led to increased ubiquitination activity in the presence of specific E1 and E2 enzymes.
It was found that expression of ORF10 also caused the downregulation of 352 proteins, while only two proteins were upregulated. Most of the downregulated proteins were found to play a role in the biogenesis, structure, and maintenance of cilia. Some of these proteins included TALPID3, TTBK2, BBS4, SEPTIN2, and IFT46.
Alarmingly, the dose-dependency studies on ORF10 revealed that higher expression of this viral protein had a greater impact on the downregulation of the aforementioned cilia proteins.
The Chinese study team then conducted several experiments on serum-starved transformed cell lines to inhibit the cellular division and allow for the formation of primary cilia. ORF10 transfection, both before and after starvation, reduced the number of cells that carried primary cilium. Taken together, these experiments similarly confirmed that ORF10 inhibits the biogenesis and maintenance of cilia. Notably, the knock-down of ZYG11B inhibited this activity of ORF10.
Significantly, the expression of ORF10 was found to significantly downregulate the ciliary Intraflagellar Transport 46 (IFT46) protein. Increased expression of IFT46 rescued ciliogenesis in cells that overexpressed ORF10, thus demonstrating that the degradation protein of this protein is involved in the mechanism by which ORF10 alters ciliogenesis.
The detailed study analysis by the French team was also published in the same peer reviewed Journal of Cell Biology.
https://rupress.org/jcb/article-standard/221/7/e202206023/213276/A-close-shave-How-SARS-CoV-2-induces-the-loss-of
The Chinese study findings also found that when the IFT46 protein lacking the C2 domain was overexpressed in ORF10-expressing cells, cilia biogenesis was partially recovered.
Importantly, this finding indicates that a completely functional IFT46 protein is not necessarily needed for cilium recovery during SARS-CoV-2 infection.
In the study, the in vivo lentiviral vector transfer of ORF10 to mice indicated that transferred ORF10 could lead to a loss of cilia within the epithelial cells that line the trachea. Notably, when mice were pseudotyped with the SARS-CoV-2 spike protein alone, this effect on the cilia was not observed, thus demonstrating that the SARS-CoV-2 spike protein is not capable of damaging cilia alone.
The Chinese study findings showed that SARS-CoV-2 targets the ubiquitin/proteasome pathway and subsequently leads to the loss of ciliary proteins. More specifically, the SARS-CoV-2 ORF10 protein is responsible for the degradation of various ciliary proteins that ultimately reduce viral particle clearance and allow for the continued spread of SARS-CoV-2 throughout the respiratory tract!
The studies illustrate yet another way for viruses to highjack the ubiquitin/proteasome pathway. In most instances reported so far, a viral protein targets a specific protein involved in intrinsic/innate defense for E3 recognition and proteasomal degradation.
The study findings demonstrate that the viral protein ORF10 induces the degradation of an array of ciliary proteins, leading to the disappearance of a whole organelle involved in viral particle clearance.
For more on the SARS-CoV-1 ORF10 and Cilia Loss, keep on logging to Thailand
Medical News.
