BREAKING! Researchers From University of North Carolina Discover That The Protein Ascl1 Can Help Heal Damaged And Failing Hearts!
Source: Cardiology Updates Oct 07, 2022 2 years, 5 months, 3 weeks, 6 days, 17 hours, 51 minutes ago
Cardiology Updates: Researchers from the McAllister Heart Institute at the University of North Carolina at Chapel Hill – USA have discovered that the protein Ascl1 which is a neuronal factor is capable of healing damaged heart muscles.
The approach of direct reprogramming has revolutionized the fields of stem cell biology and regenerative medicine.
Direct reprogramming means that reprogramming the somatic cell into a desired patient specific cell directly without passing through the pluripotent stem cell stage. This method has a low risk about epigenetic remodeling and tumor formation. Also, it is more efficient and can be accomplished in an economy of time.
The common mechanisms governing how reprogramming cells undergo transcriptome and epigenome remodeling (i.e., regulatome remodeling) however have not been properly investigated.
The study team by characterizing early changes in the regulatome of three different types of direct reprogramming, identified lineage-specific features as well as common regulatory transcription factors.
Importantly, during the process, the study team discovered that the neuronal factor Ascl1 possesses cross-lineage potential; together with Mef2c, it drives efficient cardiac reprogramming toward a mature and induced cardiomyocyte phenotype.
Utilizing ChIP-seq and RNA-seq, the study team found that MEF2C drives the shift in ASCL1 binding away from neuronal genes toward cardiac genes, guiding their co-operative epigenetic and transcription activities.
The study findings demonstrate the existence of common regulators of different direct reprogramming and argue against the premise that transcription factors possess only lineage-specific capabilities for altering cell fate ie the basic premise used to develop direct reprogramming approaches.
Most significantly, the study findings discovered that Ascl1 is able to be utilized to help heal damaged hearts by causing the generation of cardiomyocytes.
Considering that in the present COVID-19 pandemic, where heart inflammation and damage is a common occurrence along with heart failures, the discovery is a major breakthrough finding.
The study findings were published in the peer reviewed journal: Cell Stem Cell.
https://www.cell.com/cell-stem-cell/fulltext/S1934-5909(22)00383-6
The key findings of the study were:
-Comparison of direct reprogramming processes unveiled common regulators
-Ascl1 demonstrates a cross-lineage potential to activate cardiac program
-Ascl1 and Mef2c induce cardiac reprogramming with high efficiency and maturity
-Ascl1 and Mef2c cooperate to induce cardiac fate and repress neuron identity
The discovery is a significant advance in the promising field of cellular reprogramming and organ regeneration, and the discovery could play a major role in future medicines to heal damaged hearts.
The study team discovered a more streamlined and efficient method for reprogramming scar tissue cells (fibroblasts) to become healthy heart muscle cells (cardiomyocytes).
/>
Typically, fibroblasts produce the fibrous, stiff tissue that contributes to heart failure after a heart attack or because of heart disease.
For a while now, turning fibroblasts into cardiomyocytes has been investigated as a potential future strategy for treating or even someday curing common and deadly heart conditions.
To the surprise of the study team, the key to the new cardiomyocyte-making technique turned out to be a gene activity-controlling protein called Ascl1, which is known to be a crucial protein involved in turning fibroblasts into neurons.
In the past, researchers had assumed that Ascl1 was only neuron-specific.
Senior author Dr Li Qian, Ph.D., associate professor in the UNC Department of Pathology and Lab Medicine and associate director of the McAllister Heart Institute at UNC School of Medicine told specialists at Thailand Medical News’s
Cardiology Updates beat, "It's an outside-the-box finding, and we expect it to be useful in developing future cardiac therapies and potentially other kinds of therapeutic cellular reprogramming."
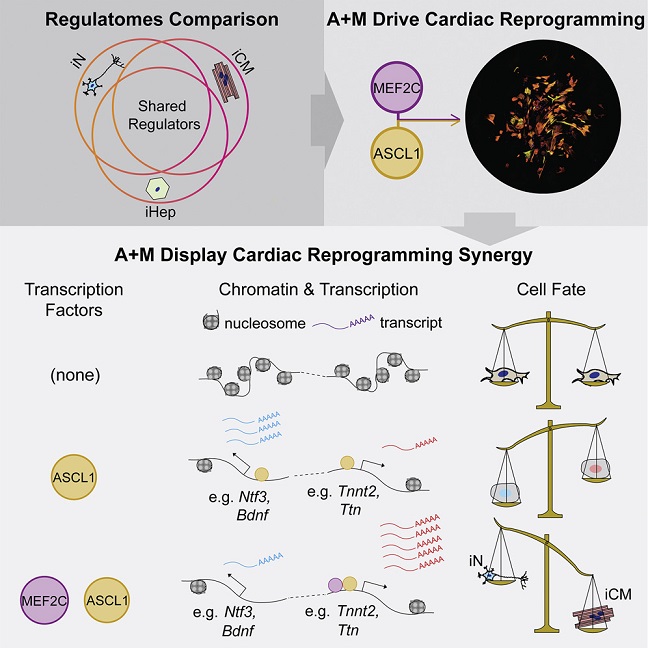 Graphical Abstract
Graphical Abstract
In the last 15 years, various scientists have developed various techniques to reprogram adult cells to become stem cells, then to induce those stem cells to become adult cells of some other type.
Researchers have even been trying to find ways to do this reprogramming more directly ie straight from one mature cell type to another, with the hope that when these methods are made maximally safe, effective, and efficient, doctors will be able to use a simple injection into patients to reprogram harm-causing cells into beneficial ones.
Dr Qian added, "Reprogramming fibroblasts has long been one of the important goals in the field. Fibroblast over-activity underlies many major diseases and conditions including heart failure, chronic obstructive pulmonary disease, liver disease, kidney disease, and the scar-like brain damage that occurs after strokes."
The study team utilized three existing techniques to reprogram mouse fibroblasts into cardiomyocytes, liver cells, and neurons. Their aim was to catalogue and compare the changes in cells' gene activity patterns and gene-activity regulation factors during these three distinct reprogrammings.
Surprisingly, the study team found that the reprogramming of fibroblasts into neurons activated a set of cardiomyocyte genes.
The study team found that this activation was due to Ascl1, one of the master-programmer "transcription factor" proteins that had been used to make the neurons.
As a result of the findings that Ascl1 activated cardiomyocyte genes, the study team added it to the three-transcription-factor cocktail they had been using for making cardiomyocytes, to see what would happen.
Shockingly, the study team found that it dramatically increased the efficiency of reprogramming ie the proportion of successfully reprogrammed cells by more than ten times!
The study findings also revealed to the study team that they could now dispense with two of the three factors from their original cocktail, retaining only Ascl1 and another transcription factor called Mef2c.
Subsequently, in further experiments the study team found evidence that Ascl1 on its own activates both neuron and cardiomyocyte genes, but it shifts away from the pro-neuron role when accompanied by Mef2c. In synergy with Mef2c, Ascl1 switches on a broad set of cardiomyocyte genes.
Dr Qian commented, "Both Ascl1 and Mef2c work together to exert pro-cardiomyocyte effects that neither factor alone exerts, making for a potent reprogramming cocktail.”
The study findings show that the major transcription factors used in direct cellular reprogramming aren't necessarily exclusive to one targeted cell type.
More importantly, the findings represent another step on the path towards future cell-reprogramming therapies for major disorders.
The study team hopes to make a two-in-one synthetic protein that contains the effective bits of both Ascl1 and Mef2c, and could be injected into failing or damaged hearts to mend them.
For the latest
Cardiology Updates, keep on logging to Thailand
Medical News.

