BREAKING! SARS-CoV-2 Causes Brain Microvascular Endothelial Cells To Become Dysfunctional Via Modulating The Wnt Signaling Pathway
Nikhil Prasad Fact checked by:Thailand Medical News Team Apr 09, 2024 1 year, 2 weeks, 3 days, 6 hours, 29 minutes ago
COVID-19 News: The COVID-19 pandemic caused by the novel coronavirus SARS-CoV-2 has led to a global health crisis, affecting millions of people worldwide. Beyond its well-known respiratory manifestations, COVID-19 has been associated with a range of neurological symptoms, including headaches, dizziness, encephalitis, and seizures. Understanding how SARS-CoV-2 interacts with the central nervous system (CNS) and impacts brain function is crucial for developing effective treatments and mitigating neurological complications. Recent research that is covered in this
COVID-19 News report by researchers from National Institute of Health Sciences, Kawasaki-Japan, Nagoya City University-Japan, National Center of Neurology and Psychiatry, Tokyo-Japan and The Jikei University Graduate School of Medicine, Tokyo-Japan, has shed light on the role of brain microvascular endothelial cells (BMECs) and the Wnt signaling pathway in mediating the effects of SARS-CoV-2 on the blood-brain barrier (BBB) and CNS function.
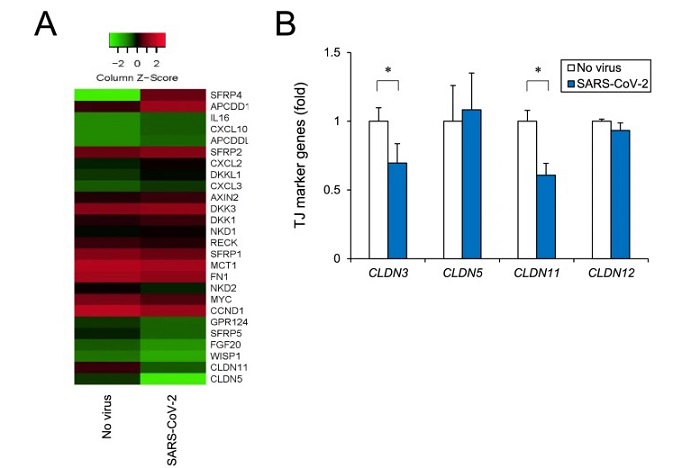 SARS-CoV-2 Causes Brain Microvascular Endothelial Cells To Become Dysfunctional Via Modulating The Wnt Signaling Pathway. Analysis of human iPSC-BMELC barrier disruption based on RNA-seq. A Comprehensive RNA-seq was performed using total RNA with or without SARS-CoV-2 infection. Heatmap shows screened up- and downregulated genes by using cut-off values of fold change of ≥ 1.5 followed by second selection based on KEGG pathway analysis and references. B After SARS-CoV-2 infection for 24 h, tight junction marker genes (CLDN3, CLDN5, CLDN11, and CLDN12) were analyzed by RT-qPCR.
The Intricacies of SARS-CoV-2 Entry and Neurological Effects
SARS-CoV-2 Causes Brain Microvascular Endothelial Cells To Become Dysfunctional Via Modulating The Wnt Signaling Pathway. Analysis of human iPSC-BMELC barrier disruption based on RNA-seq. A Comprehensive RNA-seq was performed using total RNA with or without SARS-CoV-2 infection. Heatmap shows screened up- and downregulated genes by using cut-off values of fold change of ≥ 1.5 followed by second selection based on KEGG pathway analysis and references. B After SARS-CoV-2 infection for 24 h, tight junction marker genes (CLDN3, CLDN5, CLDN11, and CLDN12) were analyzed by RT-qPCR.
The Intricacies of SARS-CoV-2 Entry and Neurological Effects
SARS-CoV-2 gains entry into host cells through the angiotensin-converting enzyme 2 (ACE2) receptor, which is expressed in various tissues, including the lungs, heart, and brain. Additionally, alternative receptors such as CD147, AXL, and Neuropilin-1 (NRP1) have been identified, hinting at the virus's ability to infect multiple cell types and organs. In the CNS, ACE2 expression in endothelial cells, including BMECs, is relatively low but still present, raising questions about the virus's potential impact on BBB integrity and CNS function.
Neurological Symptoms and BBB Disruption
COVID-19 patients commonly experience neurological symptoms, ranging from mild to severe manifestations. These symptoms can result from direct viral invasion of the CNS or secondary effects of systemic inflammation and immune responses. The BBB, composed of BMECs with tight junctions, plays a crucial role in maintaining CNS homeostasis by regulating the passage of molecules and immune cells. Disruption of the BBB, as seen in conditions like multiple sclerosis and Alzheimer's disease, can lead to increased permeability and immune cell infiltration into the brain.
Role of iPSC Technolog
y in COVID-19 Research
Human-induced pluripotent stem cells (iPSCs) offer a powerful tool for studying viral infections and their impact on human cells. iPSC-derived BMECs (iPSC-BMELCs) provide a relevant model for investigating SARS-CoV-2 infection and BBB dysfunction. Recent studies have demonstrated the susceptibility of iPSC-BMELCs to SARS-CoV-2 and the resulting changes in barrier integrity and inflammatory responses.
Experimental Findings: SARS-CoV-2 Infection of iPSC-BMELCs
In experiments using iPSC-BMELCs infected with the original strain of SARS-CoV-2, researchers observed a decrease in transendothelial electrical resistance (TEER), indicating compromised barrier function. This decrease was accompanied by downregulation of tight junction markers such as CLDN3 and CLDN11, which are essential for maintaining BBB integrity. Immunocytochemical analysis confirmed the presence of viral proteins within infected iPSC-BMELCs, highlighting the virus's ability to enter and replicate within these cells.
Inflammatory Responses and Wnt Signaling Dysregulation
SARS-CoV-2 infection of iPSC-BMELCs also triggered an inflammatory response, characterized by increased expression of proinflammatory genes and cytokines.
SARS-CoV-2 infection increased the expression levels of CCL3, CCL5, CXCL2, CXCL3, CXCL10, and IL16. ELISA confirmed the increased secretion of CXCL10, a well-known cytokine storm marker. These results suggest that SARS-CoV-2 infection induces a critical inflammatory response in BMECs.
This inflammatory cascade mirrors the systemic cytokine storm observed in severe COVID-19 cases and underscores the role of immune dysregulation in CNS complications. Importantly, RNA sequencing revealed dysregulation of the canonical Wnt signaling pathway, a critical regulator of cellular processes including inflammation and barrier integrity.
Targeting Wnt Signaling as a Therapeutic Strategy
The identification of Wnt signaling dysregulation in SARS-CoV-2-infected iPSC-BMELCs opens avenues for targeted therapeutic interventions. CHIR99021, a Wnt signaling modulator, showed promise in inhibiting viral infection and reducing inflammatory responses in these cells. By restoring Wnt signaling balance, CHIR99021 may mitigate BBB dysfunction and associated neurological symptoms in COVID-19.
Implications for COVID-19 Neurology and Treatment
The findings from iPSC-BMELC studies provide valuable insights into the pathophysiology of COVID-19-related neurological complications. The ability of SARS-CoV-2 to infect BMECs and disrupt BBB integrity highlights the potential mechanisms underlying CNS involvement in COVID-19. Targeting Wnt signaling pathways presents a novel therapeutic approach that warrants further investigation in preclinical and clinical studies.
Future Directions and Challenges
While iPSC technology offers a robust platform for studying viral infections and CNS interactions, several challenges remain. Understanding the full spectrum of SARS-CoV-2's effects on different cell types within the CNS, including neurons, astrocytes, and microglia, is essential for comprehensive treatment strategies. Additionally, translating findings from iPSC models to clinical applications requires rigorous validation and consideration of patient-specific factors.
Conclusion
In conclusion, SARS-CoV-2 infection impacts BMECs and the BBB via dysregulation of Wnt signaling, contributing to neurological symptoms observed in COVID-19 patients. iPSC technology provides a valuable tool for studying these interactions and developing targeted therapies. Continued research into the molecular mechanisms of SARS-CoV-2 neurotropism and immune responses will aid in mitigating CNS complications and improving outcomes for COVID-19 patients with neurological involvement.
The study findings were published in the peer reviewed journal: Fluids and Barriers of the CNS.
https://link.springer.com/article/10.1186/s12987-024-00533-9
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
