BREAKING! SARS-CoV-2 Causes Type I Interferon Signaling Dysregulation In Olfactory Networks With Implications Of Risk For Alzheimer’s Disease!
Nikhil Prasad Fact checked by:Thailand Medical News Team May 10, 2024 1 year, 8 months, 2 weeks, 5 days, 19 hours, 21 minutes ago
COVID-19 News: The emergence of SARS-CoV-2, the virus responsible for the COVID-19 pandemic, has brought with it a myriad of challenges beyond the acute respiratory illness it causes. One of the emerging areas of concern is its impact on neurological function, particularly cognitive impairment. Recent studies have suggested a potential link between SARS-CoV-2 infection and dysregulation of type I interferon signaling (IFN-I), a crucial component of the immune response. Furthermore, there are indications that such dysregulation may not only affect immediate cognitive function but could also have long-term implications, including an increased risk for neurodegenerative diseases like Alzheimer’s. This
COVID-19 News report delves into the intricate connections between SARS-CoV-2, IFN-I dysregulation, and their potential implications for cognitive health, particularly in the context of Alzheimer’s disease based on a study conducted by researchers from University of Cyprus, Tallaght University Hospital, Dublin- Ireland, Athens Naval Hospital-Greece and Hellenic Pasteur Institute-Greece.
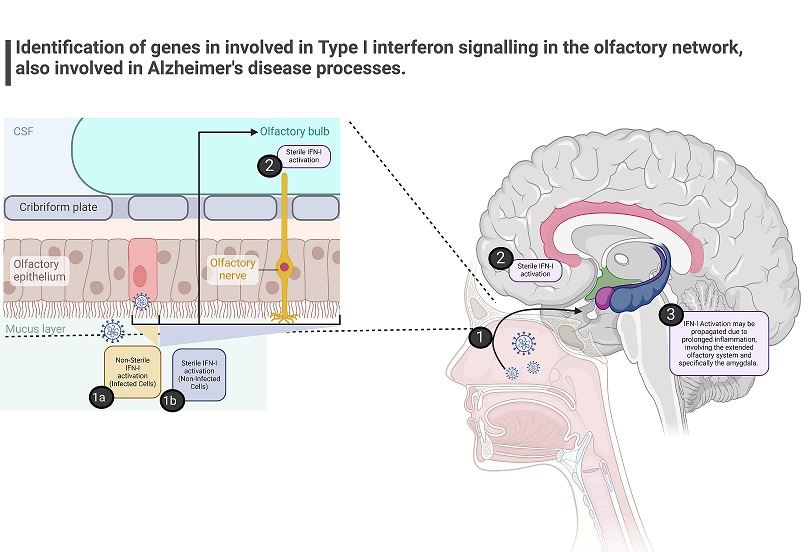 Graphical Abstract - SARS-CoV-2 Causes Type I Interferon Signaling Dysregulation In Olfactory Networks With Implications Of Risk For Alzheimer’s Disease
The Interplay of Type I Interferon Signaling and COVID-19
Graphical Abstract - SARS-CoV-2 Causes Type I Interferon Signaling Dysregulation In Olfactory Networks With Implications Of Risk For Alzheimer’s Disease
The Interplay of Type I Interferon Signaling and COVID-19
Type I interferons (IFN-I) play a critical role in the body's defense against viral infections. They are key signaling molecules that trigger a cascade of immune responses aimed at containing and eliminating pathogens. However, the dysregulation of IFN-I signaling has been identified as a significant factor in the severity and pathogenesis of COVID-19. Studies have shown that SARS-CoV-2 can disrupt IFN-I pathways, leading to an imbalance in immune responses and contributing to the diverse clinical manifestations of the disease.
Linking IFN-I Dysregulation to Cognitive Impairment
Beyond its immediate effects on the respiratory system, COVID-19 has been associated with neurological symptoms and cognitive deficits. This has prompted investigations into the mechanisms underlying these neurological manifestations. One hypothesis that has gained traction is that dysregulated IFN-I signaling, particularly in the central nervous system (CNS), could contribute to cognitive impairment observed in COVID-19 patients. This hypothesis is supported by evidence linking IFN-I dysregulation to reduced cognitive resilience and neurodegenerative processes, such as those seen in Alzheimer’s disease.
Exploring the Olfactory Route: Insights from Transcriptomic Studies
The study team focused on understanding how SARS-CoV-2 may affect the CNS through routes such as the olfactory system. The olfactory epithelium and the olfactory bulb are sites where the virus can gain entry into the CNS, potentially triggering immune responses that impact cognitive function. Transcriptomic studies have provided valuable insights into the molecular pathways involved in IFN-I dysregulation along the transolf
actory route. These studies have identified enriched IFN-I signatures and genes in nasal mucosa, olfactory bulb, and CNS tissues of COVID-19 patients, highlighting a potential link between viral infection, IFN-I responses, and cognitive pathways.
The researchers analyzed published datasets of COVID-19 patients' nasal mucosa and olfactory bulb amygdala transcriptomes to identify enriched IFN-I signatures along the transolfactory route. Surprisingly, they found significant enrichment of IFN-I pathways in all samples tested, suggesting a systemic dysregulation of IFN-I signaling in COVID-19 patients, even without direct CNS infection.
Unraveling the Molecular Networks: IFITM and OAS Families
Within the IFN-I enriched signatures, genes belonging to the IFITM (Interferon-induced transmembrane) and OAS (2'-5'-oligoadenylate synthetase) families have emerged as key players. These genes are involved in antiviral defense mechanisms and immune regulation. Interestingly, overlaps between IFN-I signatures in COVID-19 patients and those associated with Alzheimer’s disease pathology have been observed, pointing to shared molecular networks that may underlie cognitive vulnerability.
Implications for Alzheimer’s Disease Risk
A critical aspect of this research is its implications for Alzheimer’s disease risk. By analyzing gene expression data and leveraging multi-omic datasets, researchers have identified specific genes within the IFN-I signatures that are associated with increased risk for Alzheimer’s. These genes include HLA-C, HLA-B, HLA-A, PSMB8, IFITM3, HLA-E, IFITM1, OAS2, and MX1. Understanding how these genes contribute to neurodegenerative processes is crucial for developing targeted interventions and therapeutic strategies.
The Intersection of Neuroinflammation and Cognitive Resilience
Central to the discussion is the role of neuroinflammation and its impact on cognitive resilience. Dysregulated IFN-I signaling can exacerbate neuroinflammatory responses, leading to synaptic dysfunction, neuronal damage, and cognitive decline. This is particularly relevant in the context of Alzheimer’s disease, where chronic neuroinflammation is a hallmark feature. By elucidating the molecular pathways linking IFN-I dysregulation, neuroinflammation, and cognitive resilience, researchers aim to uncover potential targets for intervention and neuroprotection.
Towards Therapeutic Strategies: Targeting IFN-I Signaling
The identification of IFN-I dysregulation as a common pathway in COVID-19 and Alzheimer’s disease opens new avenues for therapeutic exploration. Strategies aimed at modulating IFN-I signaling, such as interferon beta therapy, have shown promise in preclinical studies and warrant further investigation. Additionally, the development of targeted therapies that mitigate neuroinflammation and preserve cognitive function holds potential for improving outcomes in both COVID-19 patients with neurological sequelae and individuals at risk for Alzheimer’s disease.
Conclusion
The intricate interplay between SARS-CoV-2, IFN-I dysregulation, and cognitive health underscores the complexity of COVID-19’s impact on the nervous system. By unraveling the molecular mechanisms involved, researchers are paving the way for novel diagnostic and therapeutic approaches that address not only the acute effects of the virus but also its potential long-term consequences on cognitive function and neurodegeneration. Continued interdisciplinary research and collaborative efforts are essential for advancing our understanding of these complex interactions and translating findings into clinical practice.
The study findings were published in the peer reviewed journal: Current Issues in Molecular Biology.
https://www.mdpi.com/1467-3045/46/5/277
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/scientists-discover-an-amyloidogenic-fragment-in-sars-cov-2-envelope-protein-that-promotes-serum-amyloid-a-misfolding-and-fibrillization
https://www.thailandmedical.news/news/researchers-warn-that-sars-cov-2-is-triggering-neurodegenerative-diseases-like-parkinsons
https://www.thailandmedical.news/news/study-shows-that-sars-cov-2-can-bind-to-amyloid-beta-fibrils
https://www.thailandmedical.news/news/covid-19-news-sars-cov2-nsp3-triggers-cell-death-and-exacerbates-amyloid-beta-42-mediated-neurodegeneration
https://www.thailandmedical.news/news/covid-19-new-scientist-hypothesize-that-furin-plays-a-key-role-in-the-pathogenesis-of-covid-19-associated-neurological-disorders
