BREAKING! SARS-CoV-2 Impairs Lipid Metabolic And Autophagic Pathways, Causing Damage To Heart, Liver and Kidneys. The Phytochemical Trigonelline Helps!
Source: Medical News - SARS-CoV-2 Research Apr 22, 2022 3 years, 10 months, 2 weeks, 4 hours, 46 minutes ago
A new study by researchers from the University of South Carolina-USA and Xiangya Hospital, Central South University, Hunan-China has found that the spike proteins of the SARS-CoV-2 coronavirus typically impairs the lipid metabolic and autophagic pathways in human host cells, leading to increased susceptibility to lipotoxicity via ferroptosis, which often results in the damage of cells and tissues of the heart, liver and kidneys. The study also found that an Nrf2 inhibitor such as the phytochemical Trigonelline which is an alkaloid derived from Coffee, can be used to suppress this impairment of the lipid metabolic and autophagic pathways and susceptibility to lipotoxicity.
Past studies have already showed that The COVId-19 disease causes lipid metabolic alterations in patients, but the detailed mechanism remains unknown.
The study team aimed to investigate whether the Spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) impairs lipid metabolism in human host cells.
The study team generated a Spike cell line in HEK293 using the pcDNA vector carrying the Spike gene expression cassette. A control cell line was generated using the empty pcDNA vector.
Gene expression profiles related to lipid metabolic, autophagic, and ferroptotic pathways were investigated. Palmitic acid (PA)-overload was used to assess lipotoxicity-induced necrosis.
The study findings showed that when compared with controls, the Spike cells showed a significant increase in lipid depositions on cell membranes as well as dysregulation of expression of a panel of molecules involved lipid metabolism, autophagy, and ferroptosis.
Furthermore, the Spike cells showed an upregulation of nuclear factor erythroid 2-related factor 2 (Nrf2), a multifunctional transcriptional factor, in response to PA. Furthermore, the Spike cells exhibited increased necrosis in response to PA-induced lipotoxicity compared to control cells in a time- and dose-dependent manner via ferroptosis, which could be attenuated by the Nrf2 inhibitor trigonelline. The phytochemical Trigonelline is actually an alkaloid derived from Coffee.
The study findings concluded that the Spike protein impairs lipid metabolic and autophagic pathways in host cells, leading to increased susceptibility to lipotoxicity via ferroptosis which can be suppressed by a Nrf2 inhibitor.
This new study findings also suggests a central role of Nrf2 in Spike-induced lipid metabolic impairments.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2022.04.19.488806v1
The SARS-CoV-2 virus which emerged in Wuhan-China late December 2019 and quickly spread throughout the world, causing the ongoing COVID-19 pandemic has to date according to official figures infected almost 508 million people and caused the deaths of more than 6.2 million humans. (actual figures could be as high as 4- to 5-fold!)
More than 28 months later, the pathophysiology of COVID-19 remains poorly understood.
This new study findings describes one facet of the virus-host interaction in terms of its impact on host lipid metabolism.
The SARS-CoV-2 coronavirus is primarily known for its spike glycoprotein antigens tha
t mediate host cell attachment and entry; however, this virus also produces multiple alterations in the metabolic pathways of the host cells. Thus, people with diabetes, metabolic syndrome, high cholesterol levels, obesity, and hypertension are at a higher risk of severe COVID-19 outcomes as compared to others.
Existing evidence also indicates that COVID-19 is associated with cardiovascular complications like myocardial injury and heart failure, which could be exacerbated in the presence of obesity.
Past studies reported a reduction in total and component lipoproteins in COVID-19 patients which, in turn, was associated with severe disease and fatal outcomes.
https://pubmed.ncbi.nlm.nih.gov/32320740/
https://pubmed.ncbi.nlm.nih.gov/32430154/
https://pubmed.ncbi.nlm.nih.gov/32501731/
It is already known that lipids are essential to the viral life cycle in some viruses, and lipid rafts have been implicated in the replication of SARS viruses. Thus, the current study describes the effects of the viral spike protein on host lipid synthesis, turnover, and catabolism.
The study findings showed that in contrast to controls, the spike protein was associated with impaired lipid metabolism and autophagic processes in the host cell. As a result, host cells were more readily affected by lipotoxicity, which was likely due to pathways triggered by the activation of nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated ferroptosis.
Interestingly, it was found that in cell lines expressing the spike protein, there was a greater accumulation of lipids when compared to control cells, particularly on the cell membrane. In close association with this change, which signals impaired lipid metabolism, a number of enzymes and lipid-associated proteins were upregulated. Simultaneously, other markers of autophagy and ferroptosis also exhibited altered levels of expression.
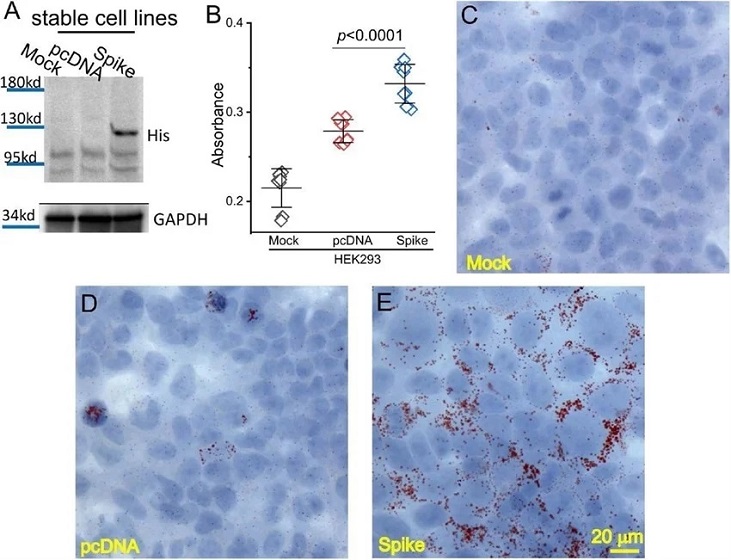 SARS-CoV-2 Spike protein caused lipid deposition in host cells. (A) Generation of Spike protein stable cell line. The expression of Spike protein was verified using an anti-His tag antibody in the Spike cells. (B) The quantification of Oil Red O staining in the mock, pcDNA, and Spike cells with measurement of an absorbance at 492 nm. (C-E) Histological images showing Oil Red O staining of lipid droplets in the mock (C), pcDNA (D), and Spike (E) cells. Nuclear components were counterstained by hematoxylin.
SARS-CoV-2 Spike protein caused lipid deposition in host cells. (A) Generation of Spike protein stable cell line. The expression of Spike protein was verified using an anti-His tag antibody in the Spike cells. (B) The quantification of Oil Red O staining in the mock, pcDNA, and Spike cells with measurement of an absorbance at 492 nm. (C-E) Histological images showing Oil Red O staining of lipid droplets in the mock (C), pcDNA (D), and Spike (E) cells. Nuclear components were counterstained by hematoxylin.
The findings showed that the spike protein negatively affected these processes within the host cell. Moreover, with an overload of palmitic acid (PA) due to abnormal lipid processing within the cell, apoptosis was induced, thus indicating the toxic effects of the lipids. While this effect was observed in all cell lines where this fatty acid accumulated, the level of severity was enhanced in spike-bearing cells.
Importantly, the effects of such an overload were dependent on both the duration of exposure and the level of PA in the cell. These cells also caused changes in the expression of some genes in response to the lipotoxicity of PA accumulation, within spike-expressing cells but not controls. In this situation, the levels of SRB1, ATG7, PTGS2, LC3 I/II ratio, and Fth1 were significantly higher as compared to controls.
Furthermore, in obesity, the cardiac muscle cells show impaired autophagy accompanied by ferroptosis that is mediated by Nrf2, the latter being the key to the changes in transcription seen in ferroptosis.
It was found that treatment with the Nrf2 inhibitor trigonelline (TRG), the ferroptosis inhibitor ferrostatin, and the PI3K inhibitor Wortmannin reduced the toxic effects of the lipid accumulation within the cells that occurred in response to the rise in PA that was enhanced in the presence of the spike protein.
It was also reported that similar findings were obtained in response to the same process within a cell line that simulated the cardiomyocyte cell line. These observations indicate the role of the spike protein in exaggerated toxicity caused by the accumulation of PA.
Corresponding author, Dr Wenbin Tan from the Department of Cell Biology and Anatomy, School of Medicine, and Department of Biomedical Engineering, University of South Carolina told Thailand
Medical News, “The research findings demonstrate that the SARS-CoV-2 spike protein has a direct role in exacerbating the toxicity of lipid accumulation within the cell as a result of the disruption of many lipid pathways in response to the viral infection. This provides some insight into why obese patients are at a greater risk of severe COVID-19 as compared to those with normal body weight.”
He further added, “Inhibitors of Nrf2, PI3K, and ferroptosis were found to reduce this toxic effect, which was observed in the form of spike-induced necrosis. Interestingly, an alkaloid called TRG, which is found at high levels in coffee, is also an effective inhibitor of lipotoxicity. This represents a potential and feasible preventive strategy to mitigate COVID-19-associated cardiometabolic pathologies associated with obesity.”
It should also be noted that PI3K is key to the autophagy of infected cells during endocytosis and part of the growth factor receptor pathways that drive the phosphorylation of viral proteins following SARS-CoV-2 infection. Hence, PI3K is a suitable target for drugs that counter or prevent COVID-19.
The SARS-CoV-2 spike protein can disrupt the expression of several PI3Ks in the host cells, thereby impairing the normal defensive response to SARS-CoV-2. This may be achieved by upregulating the formation of autophagosomes while reducing the fusion of autophagosomes with lysosomes. Autophagosomes subsequently accumulate within the cell, producing lipotoxicity.
As the drug Wortmannin, which is a steroid metabolite of the fungi Penicillium funiculosum, shows the ability to oppose this exaggerated lipotoxicity, this compound, as well as other PI3K inhibitors, could be helpful in treating COVID-19 patients with high serum lipid levels.
Nuclear factor erythroid 2-related factor 2 or Nrf2 is a regulator of over a thousand genes that participate in an array of metabolic and homeostatic pathways, such as detoxification, protein degradation, antioxidative defense, as well as iron and lipid metabolism.
Importantly, this broad functional capability of Nrf2 extends both to the adaptive capacity of the heart muscle and enhances maladaptive cardiac phenomena when the normal occurrence of myocardial autophagy is inhibited, as in chronic type 1 diabetes and obesity. This results in dysfunction of the heart musculature and abnormal remodeling.
It was found that the increase in Nrf2 expression in response to expression of the SARS-CoV-2 spike protein was mediated by PA accumulation and the resulting necrosis was suppressed by a Nrf2 inhibitor.
The stud team says that further detailed research is warranted to elucidate the exact underlying mechanisms of lipid metabolism dysregulation and determine how impaired Nrf2 signaling causes host cardiac cells in obese COVID-19 patients to experience ferroptosis induced by lipotoxicity.
For the latest
SARS-CoV-2 Research, keep on logging to Thailand
Medical News.

