BREAKING! SARS-CoV-2 Infection Induces Increase Of GP73 That Causes Dysglycaemia. Increased GP73 Could Also Imply Future Liver Disease And Liver Cancer!
Source: SARS-CoV-2-GP73 Jan 07, 2022 3 years, 11 months, 2 weeks, 3 days, 16 hours, 56 minutes ago
SARS-CoV-2-GP73: A new study led by researchers from the Beijing Institute of Biotechnology involving more than 8 other research institutions and hospitals in China has found that SARS-CoV-2 infection induces the increase of the circulating protein GP73 that causes dysglycaemia, more specifically the GP73 proteins act as a kind of glucogenic hormone contributing to SARS-CoV-2-induced hyperglycemia.

Thailand Medical News would further like to warn that based on past published studies, increase of GP73 has been linked to development of liver diseases and also liver cancer specifically hepatocellular carcinoma. Hence, we can expect to witness an increase of liver disease and liver cancer in Long COVID sufferers.
To date most severe cases of SARS-CoV-2 infections and also in some cases of even asymptomatic and mild infections, are associated with elevated blood glucose levels and metabolic complications.
The molecular mechanisms for how SARS-CoV-2 infection alters glycometabolic control are however incompletely understood.
The study team found a link between the circulating protein GP73 with enhanced hepatic gluconeogenesis during SARS-CoV-2 infection.
The
SARS-CoV-2-GP73 study team first demonstrated that GP73 secretion is induced in multiple tissues upon fasting and that GP73 stimulates hepatic gluconeogenesis through the cAMP/PKA signaling pathway.
The team then subsequently demonstrated that GP73 secretion is increased in cultured cells infected with SARS-CoV-2, after overexpression of SARS-CoV-2 nucleocapsid and spike proteins and in lungs and livers of mice infected with a mouse-adapted SARS-CoV-2 strain.
Interestingly it was found that GP73 blockade with an antibody inhibits excessive glucogenesis stimulated by SARS-CoV-2 in vitro and lowers elevated fasting blood glucose levels in infected mice.
In patients with COVID-19, plasma GP73 levels are elevated and positively correlate with blood glucose levels.
The study findings suggest that GP73 is a glucogenic hormone that likely contributes to SARS-CoV-2-induced abnormalities in systemic glucose metabolism.
The study findings were published in the peer reviewed journal: Nature Metabolism.
https://www.nature.com/articles/s42255-021-00508-2
A review article on the study findings by Dr Katie C. Coate from the Division of Diabetes, Endocrinology and Metabolism, Department of Medicine, Vanderbilt University Medical Center, Nashville-USA also supported the findings of the study.
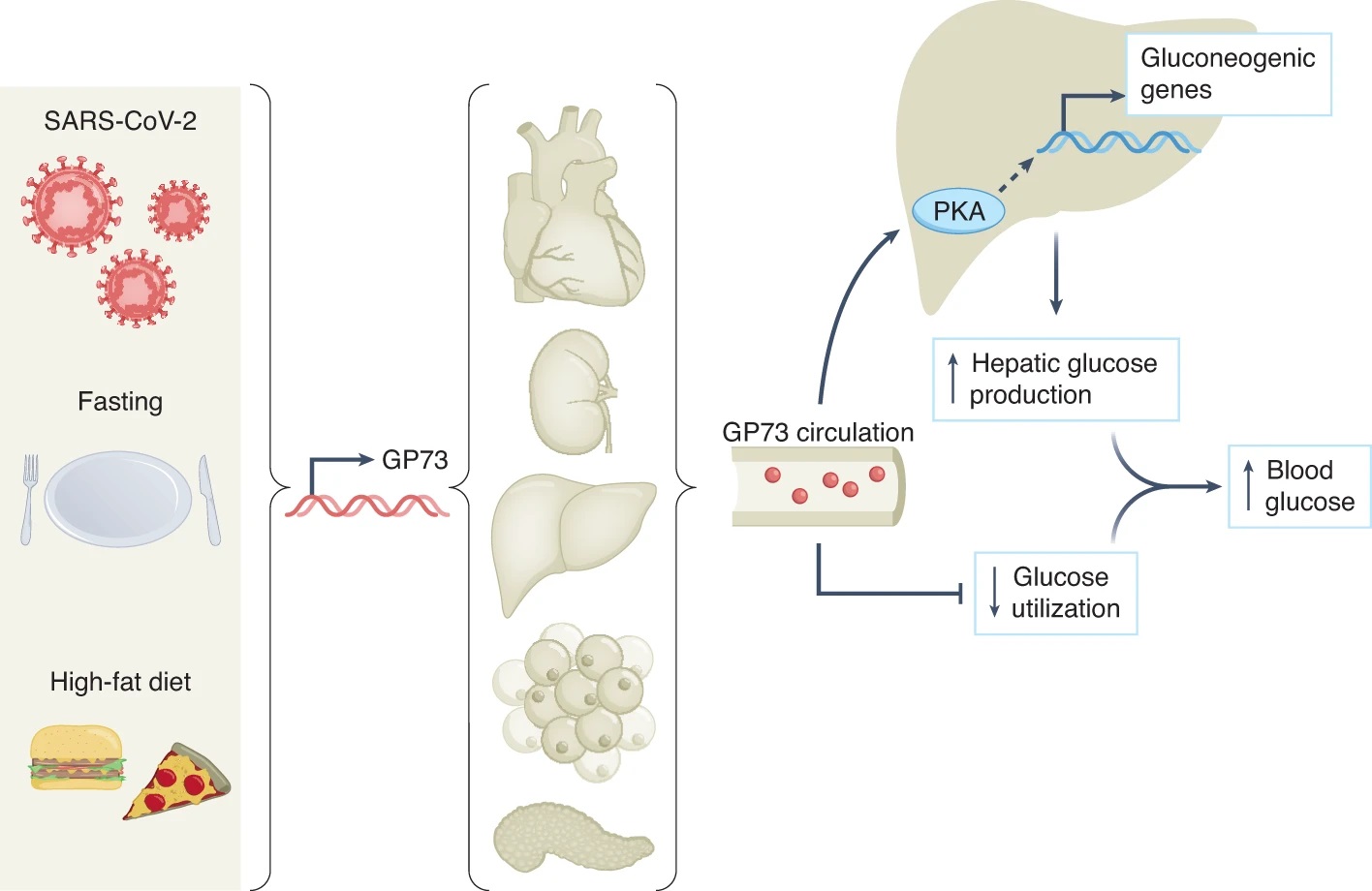 GP73 is a stress-induced secreted factor that increases hepatic glucose production, impairs whole-body glucose utilization and contributes to SARS-CoV-2-associated dysglycaemia. (Coate et Al)
GP73 is a stress-induced secreted factor that increases hepatic glucose production, impairs whole-body glucose utilization and contributes to SARS-CoV-2-associated dysglycaemia. (Coate et Al)
Different forms of metabolic stress, including SARS-CoV-2 infection, fasting or high-fat diet feeding,
induce the expression and secretion of GP73 from a variety of tissues depending on the context. Circulating GP73 travels to the liver, where it activates PKA and its downstream signalling molecules, increases the expression of gluconeogenic genes and augments hepatic glucose release. GP73 also impairs insulin-mediated glucose utilization, which, when combined with the above, elevates blood glucose.
Dr Coate said that the maintenance of glucose homeostasis in healthy individuals reflects a tightly controlled balance between whole-body glucose production and glucose utilization that is governed by a regulated network of circulating hormones and metabolites.
She said that acute and critical illness disrupts this balance and provokes hyperglycemia due to the heightened production of pro-inflammatory mediators and counter-regulatory hormones that antagonize insulin action and stimulate excessive hepatic glucose production (HGP).
The pathophysiology of hyperglycemia in the context of severe viral infection has become even more significant due to the COVID-19 pandemic, caused by the SARS-CoV-2 coronavirus.
Indeed, SARS-CoV-2 infection is often associated with elevations in blood glucose that impact disease severity and clinical outcomes, but the mechanism(s) underlying COVID-associated dysglycaemia in vivo, the target tissue(s) involved and the chronicity of disrupted glycemic control following resolution of acute illness are poorly understood.
Fortunately, the news study findings by the Chinese researchers from the Beijing Institute of Biotechnology fill an important gap in the field by identifying a new function for Golgi protein 73 (GP73) as a stress-induced secreted factor that elevates blood glucose and is required for SARS-CoV-2 associated dysglycaemia.
Dr Coates review article was also published in the peer reviewed journal: Nature Metabolism.
https://www.nature.com/articles/s42255-021-00511-7
Numerous past studies have linked SARS-CoV-2 infection with new-onset diabetes.
https://pubmed.ncbi.nlm.nih.gov/32530585/
https://pubmed.ncbi.nlm.nih.gov/32879473/
The mechanisms underlying this phenomenon are likely complex and may include the promotion of inflammation, structural lung damage and systemic effects.
It has also been noted that SARS-CoV-2 infection was associated with insulin resistance via pathways involved in endogenous glucose production.
https://pubmed.ncbi.nlm.nih.gov/33849817/
The new study findings by the Chinese researchers demonstrate that enhanced gluconeogenic metabolism following SARS-CoV-2 infection was primarily dependent on GP73.
In patients with COVID-19, as opposed to significantly elevated GP73 levels in patients with severe disease, serum GP73 levels in patients with mild disease were comparable to those observed in healthy controls, suggesting the presence of a threshold of disease severity or chronicity required for GP73 upregulation. (It should be noted however that some asymptomatic and mild COVID-19 patients did however also exhibit high elevated levels of GP73!)
Though the SARS-CoV-2 N and S proteins stimulated GP73 secretion, the study findings raise the question of whether GP73 expression is limited to SARS-CoV-2 replication.
Worryingly, the levels of GP73 remained elevated after recovery. It has been observed that hyperglycemia persists for 3 years after recovery from SARS and can be detected for at least 2 months in patients recovered from COVID-19.
https://pubmed.ncbi.nlm.nih.gov/34035524/
https://pubmed.ncbi.nlm.nih.gov/19333547/
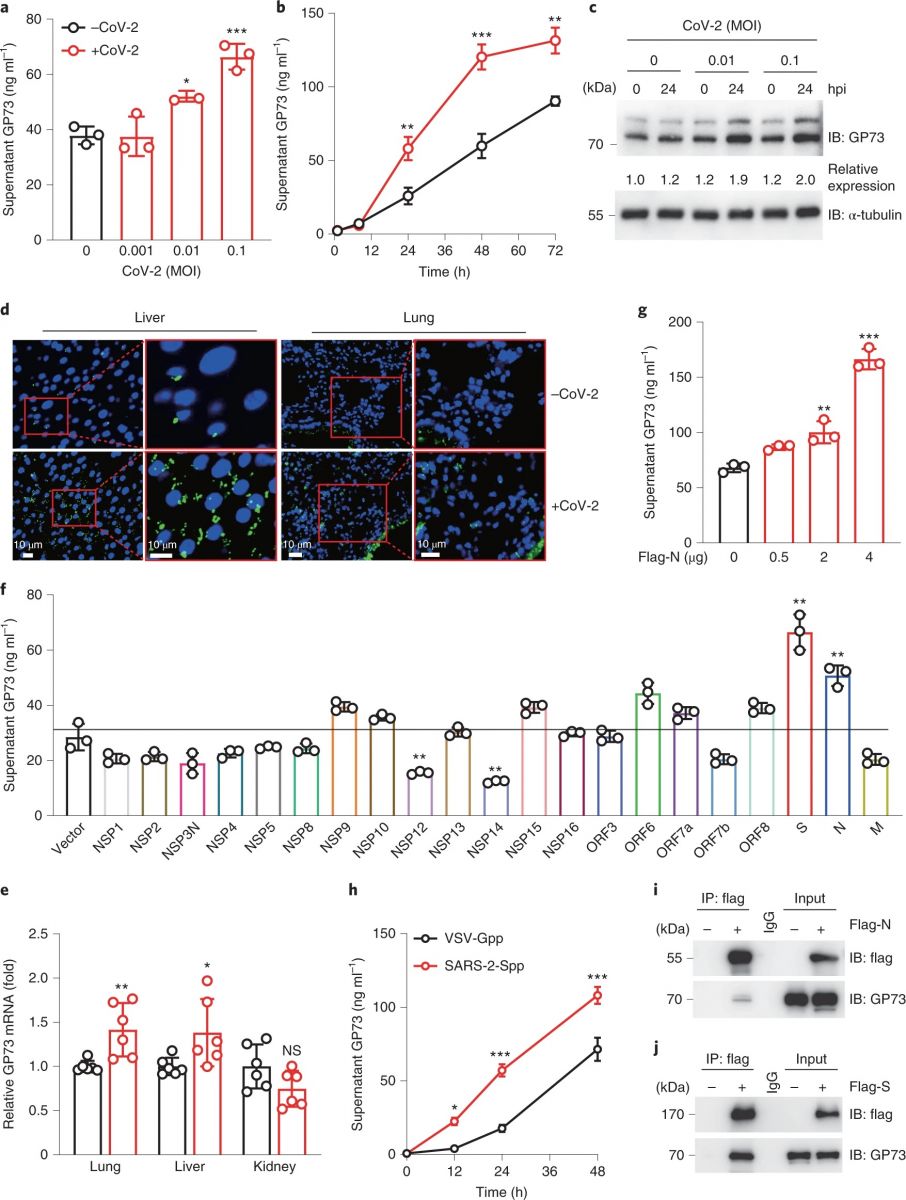 SARS-CoV-2 infection promotes GP73 production and secretion. (Wan et al)
SARS-CoV-2 infection promotes GP73 production and secretion. (Wan et al)
a,b, Supernatant GP73 levels in Huh-7 cells infected with SARS-CoV-2 at the indicated multiplicity of infection (MOI) for 24 h (a) or with SARS-CoV-2 (MOI 0.1) for the indicated times (b). P = 0.0177 for 0.01 MOI versus 0 MOI, P = 0.0002 for 0.1 MOI versus 0 MOI (a), P = 0.0049 for 0.1 MOI versus 0 MOI (24 h), P < 0.0001 for 0.1 MOI versus 0 MOI (48 h), P = 0.0010 for 0.1 MOI versus 0 MOI (72 h) by two-way ANOVA followed by Bonferroni’s post hoc test. c, Intracellular GP73 levels in Huh-7 cells infected with SARS-CoV-2 (CoV-2) at the indicated MOIs for 24 h. d, Representative of three confocal immunofluorescence images of GP73 staining in liver and lung sections from three MASCp6-infected mice. Green represents GP73 and blue represents the nucleus. e, GP73 mRNA expression in lung, liver and kidney tissues from mice inoculated with 1.6 × 104 plaque-forming units (p.f.u.) of MASCp6 and killed at day 2 after inoculation (n = 6). P = 0.0079 for infected versus mock (lung), P = 0.0389 for infected versus mock (liver) by two-tailed Student’s t-tests. f,g, Supernatant GP73 levels in Huh-7 cells transfected with plasmids expressing the indicated SARS-CoV-2 proteins (f) or different concentrations of Flag-N (g). P = 0.0097 for NSP12 versus vector, P = 0.0044 for NSP14 versus vector, P = 0.0012 for S versus vector and P = 0.0031 for N versus vector by two-tailed Student’s t-tests (f), P = 0.0036 for 0.5 μg versus 0 μg and P < 0.0001 for 4 μg versus 0 μg by one-way ANOVA followed by Bonferroni’s post hoc test (g). h, Supernatant GP73 levels in Huh-7 cells challenged with SARS-CoV-2pp or VSV-Gpp for the indicated times. P = 0.0205 for SARS-2-Spp versus VSV-Gpp (12 h), P = 0.0004 for SARS-2-Spp versus VSV-Gpp (24 h) and P = 0.0006 for SARS-2-Spp versus VSV-Gpp (48 h) by two-way ANOVA followed by Bonferroni’s post hoc test. i,j, Immunoprecipitation analysis of GP73 interaction with N (i) or S (j) protein in 293T cells transfected with Flag-N or Flag-S. Animal and cell-based studies were performed independently at least three biological replicates with comparable results. Data are mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001.
A past study showed that GP73 production is activated and correlated with interferon-β activation during RNA viral infection through MAVS.
https://pubmed.ncbi.nlm.nih.gov/28394926/
Interestingly potential mechanisms for the regulation of GP73 expression demonstrated the involvement of cytokines, liver damage and mTOR signaling.
https://pubmed.ncbi.nlm.nih.gov/25980751/
https://pubmed.ncbi.nlm.nih.gov/23144154/
https://pubmed.ncbi.nlm.nih.gov/21663469/
Hence, GP73 expression may be controlled in SARS-CoV-2-dependent and SARS-CoV-2-independent manners.
It should be noted that HFD and HBV/HCV infection also induce circulating GP73 levels.
https://pubmed.ncbi.nlm.nih.gov/12029628/
https://pubmed.ncbi.nlm.nih.gov/25314080/
Importantly as a Golgi-resident protein exhibiting glucoregulatory actions, the potential contribution of GP73 to glucose abnormalities in the context of infection with other viruses, or in metabolic diseases, including diabetes, warrants further investigation.
Apart from the role of GP73 in promoting cell proliferation, tumor development and metastasis, GP73 also represses the host innate immune response to promote RNA virus replication. https://pubmed.ncbi.nlm.nih.gov/28394926/
https://pubmed.ncbi.nlm.nih.gov/33343966/
Thailand Medical News would also like to add that numerous other studies have also linked elevated levels of GP73 to various types of liver cancer including hepatocellular carcinoma.
https://www.sciencedirect.com/science/article/pii/S2542568420300520
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC5604171/
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC3484483/
https://www.cancerjournal.net/article.asp?issn=0973-1482;year=2018;volume=14;issue=6;spage=1239;epage=1244;aulast=Jiao
https://www.journal-of-hepatology.eu/article/S0168-8278%2805%2900425-3/fulltext
Hence it is very important and critical for long-term follow-up of liver issues and cancer in infected COVID-19 patients and the need for frequent health screenings and checkups.
The study team was unable to retrieve more clinical data including information on body mass index, steatohepatitis and fibrosis status from the current patient cohort due to the urgent circumstance of the COVID-19 pandemic.
Though all patients in the study were HBV, HCV and HIV negative, the possibility that NASH might have influenced GP73 release cannot be fully excluded.
The study team concluded, “In summary, we provide evidence that SARS-CoV-2 infection induces GP73 production and secretion, which results in an exaggerated gluconeogenic response. Sustained elevated GP73 may directly predispose the host to abnormal glucose metabolism. Our findings suggest that neutralizing plasma GP73 might be therapeutic option for treating patients with SARS-CoV-2 infection that warrants further investigation.”
Thailand Medical News is currently conducting its own research on phytochemicals extracted from the plants Rumex Vesicarius, Cassia Fistula Teucrium Oliverianum and Rhazya Stricta and also phytochemicals especially the terpenoids from Grape Seed Extract and the flavonoid Naringenin from Grapefruit along-side other herbs and phytochemicals to develop a tea brew that can regulate and suppress GP73 production, to be added to the various range of teas we have developed for various long COVID issues. Readers who wish to support our cause can donate and will also be entitled to the future tea range once we are ready.
https://www.thailandmedical.news/p/sponsorship
(Minimum donations are US$100 and please do send us an email with your name, address and contact number to therapeuticteas19@gmail.com)
For the latest on
SARS-CoV-2-GP73, keep on logging to Thailand Medical News.

 GP73 is a stress-induced secreted factor that increases hepatic glucose production, impairs whole-body glucose utilization and contributes to SARS-CoV-2-associated dysglycaemia. (Coate et Al)
GP73 is a stress-induced secreted factor that increases hepatic glucose production, impairs whole-body glucose utilization and contributes to SARS-CoV-2-associated dysglycaemia. (Coate et Al)