BREAKING! SARS-CoV-2 Nucleocytoplasmic Shuttling, Nuclear Localization And Production Of Viral-Host Chimeric Proteins Causes Long COVID Issues!
COVID-19 News : Long COVID Jun 07, 2023 1 year, 10 months, 1 week, 3 days, 18 hours, 35 minutes ago
A Must Read… Detailing How The SARS-CoV-2 Virus Is Able To Enter The Host Nucleus And Produce New Viral-Host Chimeric Proteins That Serve As Neo-Antigens!
COVID-19 News: The COVID-19 pandemic caused by the SARS-CoV-2 virus has shaken the world with its devastating impact. Initially thought to primarily affect the respiratory system, it has become clear that the consequences of this virus extend far beyond that. Not only does it lead to a prolonged form of illness known as Long COVID, but recent breakthrough research by researchers from Uruguay has uncovered a fascinating aspect of SARS-CoV-2's behavior within human cells.
Traditionally, it was believed that the replication of this single-stranded RNA virus occurred solely in the cytoplasm, with no significant involvement of the host cell nucleus. However, accumulating evidence from this news study now reveals that SARS-CoV-2 components disrupt the transport of specific proteins through the nuclear pores, and is able to localize to the host nucleus, challenging the established understanding of its lifecycle.
Past studies by the U.S. NIH have also confirmed that the SARS-CoV-2 spike proteins and mRNAs can translocate to the nucleus of the host cells!
https://www.thailandmedical.news/news/breaking-u-s-nih-study-finds-that-sars-cov-2-spike-proteins-and-mrnas-can-translocate-into-the-nucleus-of-host-cells-unlike-any-other-coronaviruses
Previous
COVID-19 News reports have already showed that the SARS-CoV-2 virus is able cause host DNA damage.
https://www.thailandmedical.news/news/breaking-news-new-research-by-mit-and-nci-provides-further-evidence-of-controversial-claims-that-sars-cov-2-genes-can-integrate-with-human-dna
https://www.thailandmedical.news/news/breaking-university-of-vermont-scientists-confirms-that-sars-cov-2-has-ability-to-damage-human-dna-and-telomeres-causing-long-covid-issues
https://www.thailandmedical.news/news/breaking-study-discovers-sars-cov-2-could-be-carcinogenic-as-it-causes-mutagenesis,-telomere-dysregulation-and-impairs-dna-mismatch-repair
host-protein-pcna-for-replication-while-dna-of-the-host-cells-are-damaged">https://www.thailandmedical.news/news/breaking-brazil-researchers-validates-that-sars-cov-2-utilizes-human-host-protein-pcna-for-replication-while-dna-of-the-host-cells-are-damaged
https://www.thailandmedical.news/news/great-news-study-shows-that-sars-cov-2-protein-nsp13-is-able-to-cause-dna-damage-and-dysregulate-tumor-suppressor-gene-p53,-increasing-cancer-risk
https://www.thailandmedical.news/news/breaking-university-of-queensland-study-discovers-that-sars-cov-2-actually-attacks-the-dna-of-heart-cells
https://www.thailandmedical.news/news/sars-cov-2-research-orf6-and-nsp13-proteins-cause-degradation-of-the-dna-damage-response-kinase-chk1-through-proteasome-and-autophagy
https://www.thailandmedical.news/news/pathological-effects-of-sars-cov-2-on-the-cell-nucleus-and-their-implications-in-long-covid
Remarkably, certain structural proteins of SARS-CoV-2, including Spike (S) and Nucleocapsid (N), as well as non-structural proteins like Nsp1 and Nsp3, and some accessory proteins (ORF3d, ORF6, ORF9a), possess the ability to enter the nucleus.
They achieve this through nuclear localization signals (NLS) or by hitchhiking with other proteins in the cell's shuttling machinery. Additionally, a portion of the viral RNA can also reach the nucleoplasm.
Intriguingly, recent findings have raised controversy by demonstrating that SARS-CoV-2 sequences can, under certain conditions, be retrotranscribed and integrated into the host genome as DNA, resulting in the formation of chimeric genes. This phenomenon implies that the virus may produce viral-host chimeric proteins within infected cells. These newly formed proteins have the potential to act as neo-antigens, triggering autoimmunity and promoting a chronic pro-inflammatory state in affected individuals.
The nucleus of a cell is responsible for storing and regulating genetic material, making it a vital component of cellular function. The transport of molecules between the nucleus and cytoplasm occurs through nuclear pores, which are composed of various proteins known as nucleoporins. SARS-CoV-2 has been found to exploit these transport mechanisms, interfering with the normal flow of proteins and genetic material.
Previous studies have shown that related viruses, such as SARS-CoV and MERS, interact with specific importins, impeding the nuclear translocation of crucial proteins involved in the antiviral response. Similarly, SARS-CoV-2 interacts with a multitude of nucleoporins and nuclear transport receptors, disrupting the nuclear import and export processes. For instance, the viral protein ORF6 inhibits the activation of important transcription factors like IRF3 and STAT1 by interacting with specific nucleoporins. This interference with nuclear translocation enables the virus to evade the host's immune response.
Moreover, the disruption of nuclear transport processes by SARS-CoV-2 not only impacts the host immune response but also affects various cellular functions. The nucleus serves as the control center of the cell, regulating gene expression and coordinating essential cellular processes. By interfering with nuclear transport, the virus can manipulate the host cell's machinery to its advantage.
One of the critical cellular processes affected by the disruption of nuclear transport is the regulation of gene expression. The transport of transcription factors and other regulatory proteins in and out of the nucleus is vital for the proper control of gene expression. SARS-CoV-2's interference with nuclear transport can lead to dysregulation of gene expression, resulting in altered cellular responses and favoring viral replication and survival.
In addition to gene expression regulation, nuclear transport disruption can also impact other important cellular activities. For example, the nucleocytoplasmic shuttling of proteins involved in DNA repair is essential for maintaining genome stability. The impairment of nuclear transport by SARS-CoV-2 can compromise the DNA repair machinery, increasing the susceptibility of infected cells to DNA damage and genomic instability.
Furthermore, the nucleus plays a crucial role in coordinating cellular signaling pathways. Signaling molecules often need to translocate between the nucleus and the cytoplasm to transmit signals and activate appropriate cellular responses. By interfering with nuclear transport, SARS-CoV-2 can disrupt these signaling pathways, leading to aberrant cellular signaling and promoting viral replication.
The disruption of nuclear transport by SARS-CoV-2 can have implications for the integrity and function of the host cell's nuclear envelope. The nuclear envelope is a double membrane structure that separates the nucleus from the cytoplasm and maintains the compartmentalization of nuclear and cytoplasmic contents. Proper nuclear transport is crucial for maintaining the integrity and functionality of the nuclear envelope.
The nuclear envelope contains specialized nuclear pore complexes (NPCs) that act as gatekeepers for the transport of macromolecules between the nucleus and the cytoplasm. The interactions between viral proteins and nucleoporins can lead to alterations in the structure and function of NPCs, affecting their ability to selectively transport proteins and RNAs. This disruption can compromise the nuclear envelope's integrity, allowing leakage of nuclear components into the cytoplasm and perturbing cellular homeostasis.
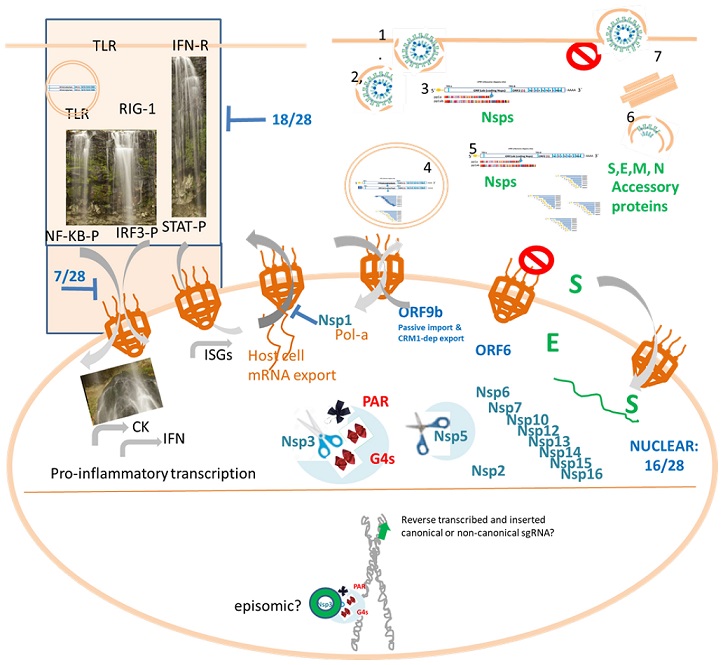 SARS-CoV-2 biology highlighting the importance of further studies of the interaction of canonical and non-canonical RNAs and proteins with host cell nucleus. Top. Canonical SARS-CoV-2 productive cycle (right) and IFN signaling interference (left). Proinflammatory signaling cascades involved in Cytokine (CK) and interferon (IFN) production, as well as IFN-dependent transcription, are hijacked by 18/28 SARS-CoV2 proteins upstream or at the transcription factor phosphorylation step while 7/28 can block the respective nucleocytoplasmic shutting of phosphorylated transctiption factors (NF-KB-P, IRF3-P or STAT-P). Bottom. The cell nucleus is represented displaying the SARS-CoV-2 proteins that have been detected in the nucleus, which constitute 16/28 proteins. Besides, a chromosome cartoon remembers us the episomic hypothesis to achieve persistence and the fact that at least under L1 overexpression, retrotranscribed copies of SARS-CoV2 sgRNAs is claimed to be inserted in the human genome.
SARS-CoV-2 biology highlighting the importance of further studies of the interaction of canonical and non-canonical RNAs and proteins with host cell nucleus. Top. Canonical SARS-CoV-2 productive cycle (right) and IFN signaling interference (left). Proinflammatory signaling cascades involved in Cytokine (CK) and interferon (IFN) production, as well as IFN-dependent transcription, are hijacked by 18/28 SARS-CoV2 proteins upstream or at the transcription factor phosphorylation step while 7/28 can block the respective nucleocytoplasmic shutting of phosphorylated transctiption factors (NF-KB-P, IRF3-P or STAT-P). Bottom. The cell nucleus is represented displaying the SARS-CoV-2 proteins that have been detected in the nucleus, which constitute 16/28 proteins. Besides, a chromosome cartoon remembers us the episomic hypothesis to achieve persistence and the fact that at least under L1 overexpression, retrotranscribed copies of SARS-CoV2 sgRNAs is claimed to be inserted in the human genome.
The disruption of nuclear transport by SARS-CoV-2 can have implications for cellular stress responses and apoptosis. The nucleus contains sensors that monitor cellular stress and initiate appropriate responses, such as activating stress-responsive transcription factors or triggering programmed cell death (apoptosis) when necessary. Dysregulation of nuclear transport can impair the activation of stress response pathways and compromise the cell's ability to mount an effective defense against viral infection.
Nuclear transport disruption by SARS-CoV-2 may have long-term consequences for the infected individuals. Persistent viral infections or chronic inflammation resulting from the impaired immune response can contribute to the development of various diseases. For example, chronic inflammation has been associated with an increased risk of cardiovascular diseases, neurodegenerative disorders, and certain types of cancers. Understanding the long-term effects of nuclear transport disruption by SARS-CoV-2 is an active area of research to identify potential strategies for mitigating these effects and promoting recovery in individuals affected by COVID-19.
The Spike protein of SARS-CoV-2 has unique features, including HIV-like inserts and a furin-cleavable site, which facilitate viral entry and spreading. It also contains a nuclear localization signal (NLS) and has been detected in the nucleus of infected cells. This nuclear localization may have implications for transcriptional regulation and phenotypic changes in host cells expressing Spike protein.
SARS-CoV-2 RNA has been detected in the nucleus of infected cells, along with the Spike protein. This nuclear translocation of viral RNA is a novel feature of SARS-CoV-2. Additionally, evidence suggests that SARS-CoV-2 RNA can be reverse-transcribed and integrated into the DNA of infected human cells, although the frequency of such events is low. Integration of viral sequences into the host genome may have implications for prolonged PCR positivity after infection.
There are some limitations and controversies surrounding the detection and significance of SARS-CoV-2 integration into the host genome. The methods used to detect chimeric viral-host RNA transcripts are prone to artifacts, and the abundance and structure of integrated SARS-CoV-2 sequences are still uncertain.
In summary, the interaction between SARS-CoV-2 and the host cell's nuclear transport machinery and its ability to localize to the nucleus of infected cells, potentially impacting host cell functions and transcriptional regulation, represents a crucial aspect of viral pathogenesis. By disrupting nuclear transport processes, the virus can evade the immune response, manipulate cellular functions, and promote its own replication and survival. Understanding the molecular mechanisms underlying this disruption can aid in the development of targeted therapeutic interventions to restore normal cellular functions, enhance the immune response, and mitigate the severity of COVID-19.
The study findings were published in the peer reviewed journal: Pathogens.
https://www.mdpi.com/2076-0817/12/6/806
(We strongly advise all to read this study in detail as even we could not cover all aspects in our review.)
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Must Read:
https://www.thailandmedical.news/news/covid-19-news-harvard-and-mit-study-alarmingly-shows-that-sars-cov-2-rna-integrates-into-human-genome-with-varying-implications
https://www.thailandmedical.news/news/urgent-studies-needed-to-assess-if-sars-cov-2-can-cause-missense-mutations-in-human-dna-and-give-rise-to-new-medical-conditions-never-seen-before
https://www.thailandmedical.news/news/breaking-swedish-study-shows-that-sars-cov-2-causes-epigenetic-changes-to-various-genes-in-human-host
https://www.thailandmedical.news/news/breaking-chinese-scientists-discover-that-sars-cov-2-causes-dysregulation-of-host-mirnas-and-also-produces-novel-mirnas,-resulting-in-varying-clinical
https://www.thailandmedical.news/news/breaking-study-discovers-that-sars-cov-2-orf8-encoded-protein-contains-a-histone-mimic-that-disrupts-human-host-cell-epigenetic-regulation
https://www.thailandmedical.news/news/breaking-singapore-study-uncovers-that-sars-cov-2-alters-human-host-rna-to-improve-viral-fitness
https://www.thailandmedical.news/news/breaking-university-of-texas-study-discovers-that-sars-cov-2-alters-human-host-chromatin-complex-to-cause-immune-dysfunction

