BREAKING! Scientists Warns That SARS-CoV-2 Also Interacts With IGF2BP1- An Oncofetal RNA-Binding Protein That Typically Fuels Tumor Virus Propagation!
Medical News - SARS-CoV-2 - IGF2BP1 Jun 29, 2023 1 year, 9 months, 2 weeks, 1 day, 19 hours, 12 minutes ago
The study found that the SARS-CoV-2 virus stimulates the expression of IGF2BP1 RNA upon infection!
Medical News: A new study conducted by the Institute of Molecular Medicine at Martin Luther University Halle-Wittenberg in Germany has revealed a new mechanism by which the SARS-CoV-2 virus interacts with a protein called IGF2BP1, known to be involved in tumor growth. This discovery sheds light on the intricate ways in which viruses exploit host cell components to enhance their own propagation. But the study can also have alarmin implications in terms of long term health issues of those who have been exposed to the SARS-CoV-2!
IGF2BP1, also known as IMP-1, CRD-BP, and VICKZ1, is an oncofetal RNA-binding protein that plays a crucial role in regulating tumor and stem cell fate. Elevated levels of IGF2BP1 have been associated with the development and progression of various cancers, including ovarian, lung, pancreatic, liver, and breast cancers, and are typically indicative of poor prognosis. The protein functions by protecting its target RNAs, which are predominantly pro-oncogenic, from degradation by microRNAs (miRNAs), thereby promoting their expression.
Recent evidence suggests that certain viruses, such as hepatitis B/C and human papillomaviruses, exploit IGF2BP1 to enhance their own propagation.
Corresponding author Dr Stefan Hüttelmaier, a professor at the Institute of Molecular Medicine, Martin Luther University Halle-Wittenberg told Thailand
Medical News, “
Surprisingly, the study also found that non-oncogenic viruses like SARS-CoV-2 also take advantage of IGF2BP1. The only virus known to be inhibited by IGF2BP1 to date is HIV-1.”
The study reviewed existing knowledge about the interactions between IGF2BP1 and various virus species. It analyzed publicly available high-throughput datasets to present a comprehensive summary of these interactions. The research indicates that viruses actively reprogram the metabolism of host cells to support infection and escape or suppress host defense mechanisms. This metabolic reprogramming, similar to the changes observed in tumor development, has been linked to viral oncogenesis. Several viruses, including Epstein–Barr virus, human papillomaviruses, hepatitis B and C viruses, human T lymphotropic virus, and Merkel cell polyomavirus, possess oncogenic potential in humans. Moreover, non-human viruses like simian virus 40 (SV40) and adenoviruses have been shown to transform animal and human cells.
RNA-binding proteins (RBPs), such as IGF2BP1, play a vital role in regulating both host and viral RNA through post-transcriptional gene regulation. Additionally, miRNAs, small non-coding RNAs that control mRNA targets, are also involved in regulating viral RNAs. The study reveals that virus infections induce post-transcriptional modifications on host genes' transcripts, including the most prevalent modification, N6-adenosine methylation (m6A). This modification affects RNA structure and function and can alter viral production and infectivity. However, the impact of m6A modifications on viral RNA varies depending on the virus species.
r />
Regarding the interaction between IGF2BP1 and SARS-CoV-2, the study found that the virus stimulates the expression of IGF2BP1 RNA upon infection. Knockdown or knockout of IGF2BP1 in different cell lines resulted in reduced levels of viral RNA, indicating that IGF2BP1 plays a role in promoting SARS-CoV-2 replication. Notably, IGF2BP1 was shown to bind and stabilize a specific subgenomic RNA encoding the SARS-CoV-2 spike (S) protein, which is critical for viral entry into host cells.
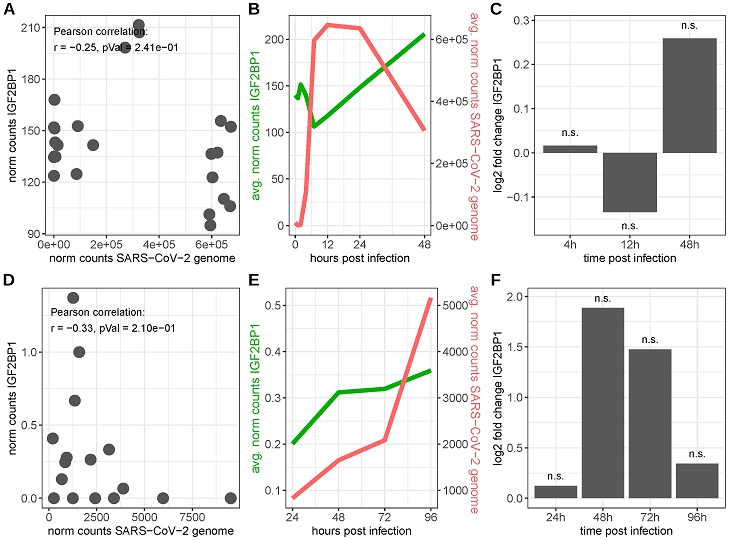
IGF2BP1 RNA expression in SARS-CoV-2-infected cells. Upper row: SARS-CoV-2-infected Caco-2 cells (data derived from GEO series GSE217504), lower row: SARS-CoV-2-infected primary human bronchial epithelial cells (HBECs, data derived from [119]). (A,D) RNA expression levels of IGF2BP1 and the SARS-CoV-2 genome in SARS-CoV-2-infected cells. (B,E) Time-point-averaged RNA expression levels of IGF2BP1 and the SARS-CoV-2 genome at the indicated time after infection. (C,F) IGF2BP1 fold changes in SARS-CoV-2 infected cells compared to mock-infected (Caco-2)/uninfected (HBECs) cells. n.s.: FDR adjusted p-value ≥ 0.05.
The study also analyzed publicly available RNA-sequencing data from SARS-CoV-2-infected cell lines and found considerable variation in viral RNA levels among infected cell lines could be attributed, at least in part, to the differential expression of IGF2BP1. Cell lines with higher levels of IGF2BP1 exhibited increased viral RNA levels, while those with lower IGF2BP1 expression showed reduced viral RNA levels.
Further investigations into the molecular mechanisms underlying the interaction between SARS-CoV-2 and IGF2BP1 demonstrated that IGF2BP1 promotes viral RNA stability by protecting the viral RNA from degradation. This stabilization effect was observed specifically on the subgenomic RNA encoding the spike protein, which is crucial for viral entry into host cells. The study also revealed that IGF2BP1 enhances the translation efficiency of the viral RNA, leading to increased production of viral proteins.
These findings have important implications for our understanding of SARS-CoV-2 infection and its potential link to tumor biology. By exploiting IGF2BP1, the virus can enhance its own replication and propagation within host cells. This mechanism may contribute to the observed variations in viral load and disease severity among individuals infected with SARS-CoV-2.
Moreover, the study highlights the complex interplay between viral infections and host cell components involved in oncogenesis. The shared utilization of IGF2BP1 by both oncogenic and non-oncogenic viruses suggests a convergence of molecular strategies employed by different viruses to manipulate host cellular processes for their own benefit.
The identification of IGF2BP1 as a key player in SARS-CoV-2 infection opens up new possibilities for therapeutic interventions. Targeting IGF2BP1 or its associated pathways could potentially limit viral replication and reduce the severity of COVID-19. However, further research is needed to fully understand the precise mechanisms by which IGF2BP1 interacts with SARS-CoV-2 and to explore the potential therapeutic implications of these findings.
The study findings were published in the peer reviewed journal: Viruses.
https://www.mdpi.com/1999-4915/15/7/1431
For the latest Medical News, keep on logging to Thailand Medical News.

