BREAKING! U.S. NIH Study Reveals Weak Antibody Response Following BA.2 infection, Paving The Way For Extended Community Circulation Due To Reinfections!
Source: Medical News - BA.2 Antibody Response Apr 13, 2022 3 years, 9 months, 11 hours, 55 minutes ago
A new study funded by the U.S. NIH that involved researchers from TyrolPath Obrist Brunhuber GmbH-Austria, National Institutes of Health, Bethesda-USA and Georgetown University, Washington, DC-USA has found that antibody response following Omicron BA.2 infection is lower than BA.1.
The study team warns that the lower neutralization response following BA.2 infection might contribute to prolonged circulation in the population due to attenuated protection from re-infection!
The study team measured antibody titers and neutralizing antibody responses in 74 outpatients infected with either omicron BA.1 or BA.2 sub-lineages.
Although none of the patients had been vaccinated, 26 had been infected by an earlier variant.
More than 20% of the BA.1 infected, but none of the BA.2 infected patients, had moderate or severe disease. Humoral response was measured between 7- and 21-days post documented omicron BA.1 or BA.2 infection.
The study findings showed that in previously uninfected and unvaccinated individuals both anti-omicron and ancestral spike IgG titers were 4-5-fold lower following BA.2 as compared to BA.1 infection.
In contrast, antibody titers in individuals previously infected with an earlier SARS-COV-2 variant were 11-86-fold higher, with no difference in titers between those infected with the BA.1 versus BA.2 variants. For reference, titers from individuals infected with the Beta variant are shown. These approximate those seen in individuals previously infected with an earlier variant who then experienced BA.1 or BA.2 infection.
The differential response in antibody generation following BA.1 and BA.2 infection was consistent across all SARS-COV-2 variants tested.
The study team next measured neutralization capacity using the angiotensin-converting enzyme 2 (ACE2) binding inhibition assay.
Neutralizing activities against the ancestral strain and omicron were similarly low in antigen naïve individuals infected with either BA.1 or BA.2. Similar to overall antibody titers, neutralizing activity was higher in those previously infected. Activity from BA.1 and BA.2 infected individuals approximated that found for individuals infected with the Beta variant and was similar across all SARS-COV2 variants tested against.
The study findings reveal that in antigen naïve individuals the immunologic response following Omicron BA.2 infection is even lower than BA.1 infection.
The study team said, “One could postulate that the lower neutralization response following BA.2 infection might contribute to prolonged circulation in the population due to attenuated protection from re-infection. Potentially, this would be abrogated by vaccination following resolution of the omicron infection per current guidelines.”
Thailand
Medical News would like to add that there is no real concrete data that claims that vaccination can completely resolve this issue as claimed by the U.S. NIH sponsored researchers as already we are witnessing that the BA.2 is causing breakthrough infections and also reinfections even in the fully vaccinated coupled with the fact that more than 34 identified BA.2 subvariants and third generation subvariants with concerning mu
tations that are able to increase transmissibility and evade immunity are already gaining predominance in circulation out of the hundreds of emerging BA.2 subvariants!
https://www.thailandmedical.news/news/breaking-debut-of-sars-cov-2-ba-4,-ba-5-variants,-recombinant-xe-variant-and-more-than-34-other-subvariants-heralds-doomsday-scenario
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.medrxiv.org/content/10.1101/2022.04.07.22273565v1
The study team basically determined immune responses generated by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron BA.2 and BA.1 sub-lineage infections in prior virus-infected and naïve subjects.
To date, the significantly transmissible SARS-CoV-2 B.1.1.529 (Omicron) variant has supplanted the prior viral variants and is less vulnerable to neutralizing antibodies induced by coronavirus disease 2019 (COVID-19) or vaccination. Therefore, the SARS-CoV-2 Omicron BA.2 sub-lineage is currently dominant.
Many studies have reported that COVID-19 vaccinated subjects infected with the SARS-CoV-2 Omicron BA.1 sub-lineage have generated detectable neutralizing antibody levels against BA.2 and BA.1 sub-lineages. However, the capacity of BA.2 infection to elicit neutralizing antibodies in COVID-19 vaccine-naïve individuals, either with or without prior SARS-CoV-2 infection, has yet to be determined.
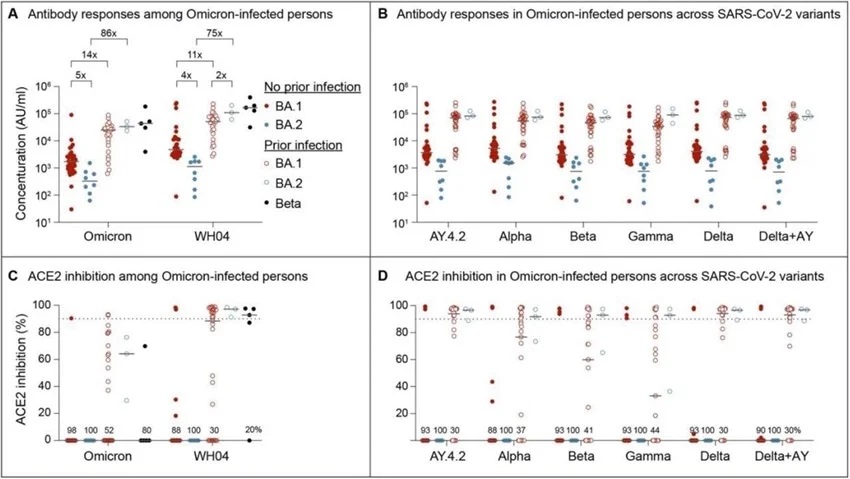 Serology and Neutralizing antibody responses in outpatients infected with the omicron BA.1 and BA.2 sublineages. Panel A and B show plasma IgG antibody binding the SARS-CoV-2 RBD (spike) from the ancestral and Omicron strains (A) as well as other variants (B) in the unvaccinated BA.1 and BA.2 Omicron patients without or with prior SARS-CoV-2 infection. Panel C and D show neutralizing antibody response by measuring inhibition of binding between ACE2 and SARS-CoV-2 spike protein. Antibody titers were measured 1-3 weeks after the infection. Results are shown as the median and dots for each data (No prior infection, BA.1, n = 40; No prior infection, BA.2, n = 10; Prior infection, BA.1, n = 23; Prior infection, BA.2, n = 3; Beta infection, n = 4).
Serology and Neutralizing antibody responses in outpatients infected with the omicron BA.1 and BA.2 sublineages. Panel A and B show plasma IgG antibody binding the SARS-CoV-2 RBD (spike) from the ancestral and Omicron strains (A) as well as other variants (B) in the unvaccinated BA.1 and BA.2 Omicron patients without or with prior SARS-CoV-2 infection. Panel C and D show neutralizing antibody response by measuring inhibition of binding between ACE2 and SARS-CoV-2 spike protein. Antibody titers were measured 1-3 weeks after the infection. Results are shown as the median and dots for each data (No prior infection, BA.1, n = 40; No prior infection, BA.2, n = 10; Prior infection, BA.1, n = 23; Prior infection, BA.2, n = 3; Beta infection, n = 4).
Hence the study team quantified the neutralizing antibody responses and antibody concentrations in the blood samples of 74 outpatients infected with the SARS-CoV-2 Omicron BA.2 or BA.1 sub-lineages between December 2021 and March 2022 in Austria. The subjects submitted written informed consent before enrolling in the study. Further, the data of the study volunteers used in this work were anonymized and coded. This research exposed participants to the least amount of risk possible.
Despite the fact that none of the study volunteers were COVID-19 vaccinated, 26 participants had a history of SARS-CoV-2 infection with a previous variant.
The study team measured the humoral responses of the study volunteers between one and three weeks after the documented Omicron BA.2 or BA.1 infections.
The study team then used antibody concentrations of the individuals infected with the SARS-CoV-2 Beta variant for comparison.
Subsequently end-point binding immunoglobulin G (IgG) antibody titers to several antigens derived from SARS-CoV-2 were quantified using the Meso Scale Discovery (MSD) technology. The V-plex multi-spot SARS-CoV-2 serology kits were used to assay the 1) SARS-CoV-2 nucleocapsid (N), spike (S), and 2) SARS-CoV-2 Beta, Alpha, Delta, Omicron, and Gamma S subdomains.
The study team further quantified the neutralization ability of the samples by performing the angiotensin-converting enzyme 2 (ACE2) binding inhibition analysis. The V-PLEX SARS-CoV-2 ACE2 neutralization kit was used to quantify antibodies that hinder the binding of ACE2 with the S subdomains of the SARS-CoV-2 variants.
The research findings demonstrated that over 20% of the SARS-CoV-2 Omicron BA.1 infected subjects had severe or moderate COVID-19, while this was not observed in BA.2 infected subjects.
Interestingly, in the SARS-CoV-2 infected and vaccination-naïve individuals, both anti-SARS-CoV-2 ancestral and Omicron S IgG concentrations were four to five times reduced after BA.2 infection relative to BA.1. On the contrary, antibody levels in COVID-19 convalescent subjects were 11 to 89-times higher, with no variation in concentrations between the BA.1 and BA.2 infected subjects.
It was also found that the antibody titers from Beta-infected subjects were similar to the COVID-19 convalescents who experienced Omicron BA.2 or BA.1 infections. Across all SARS-COV-2 variants studied, the differential response in antibody production following BA.2 and BA.1 infection was constant.
The study findings showed that neutralizing capacities against the SARS-CoV-2 Omicron and ancestral strains were comparably reduced in the previously uninfected and unvaccinated subjects infected with either the Omicron BA.2 or BA.1 sub-lineages. Identical to the overall antibody concentrations, neutralizing activity was heightened in the subjects with COVID-19 history following BA.2 or BA.1 infections. In addition, neutralizing activity in SARS-CoV-2 Beta-infected and COVID-19 convalescent BA.2- or BA.1-infected subjects was comparable, and it was consistent among all SARS-CoV-2 variants assessed.
The study findings significantly showed that the immune response after the SARS-CoV-2 Omicron BA.2 infections in the SARS-CoV-2 antigen-naïve subjects was much lower than the BA.1 infections.
The study team warns that the diminished neutralization response after BA.2 infections might promote extended circulation of BA.2 in the community due to reduced immunity against SARS-CoV-2 re-infections.
For more on
BA.2 variants and subvariants, keep on logging to Thailand Medical News.

 Serology and Neutralizing antibody responses in outpatients infected with the omicron BA.1 and BA.2 sublineages. Panel A and B show plasma IgG antibody binding the SARS-CoV-2 RBD (spike) from the ancestral and Omicron strains (A) as well as other variants (B) in the unvaccinated BA.1 and BA.2 Omicron patients without or with prior SARS-CoV-2 infection. Panel C and D show neutralizing antibody response by measuring inhibition of binding between ACE2 and SARS-CoV-2 spike protein. Antibody titers were measured 1-3 weeks after the infection. Results are shown as the median and dots for each data (No prior infection, BA.1, n = 40; No prior infection, BA.2, n = 10; Prior infection, BA.1, n = 23; Prior infection, BA.2, n = 3; Beta infection, n = 4).
Serology and Neutralizing antibody responses in outpatients infected with the omicron BA.1 and BA.2 sublineages. Panel A and B show plasma IgG antibody binding the SARS-CoV-2 RBD (spike) from the ancestral and Omicron strains (A) as well as other variants (B) in the unvaccinated BA.1 and BA.2 Omicron patients without or with prior SARS-CoV-2 infection. Panel C and D show neutralizing antibody response by measuring inhibition of binding between ACE2 and SARS-CoV-2 spike protein. Antibody titers were measured 1-3 weeks after the infection. Results are shown as the median and dots for each data (No prior infection, BA.1, n = 40; No prior infection, BA.2, n = 10; Prior infection, BA.1, n = 23; Prior infection, BA.2, n = 3; Beta infection, n = 4).