BREAKING! Worrisome Study Findings Reveal That SARS-CoV-2 Variants’ Spike Proteins Can Bind To More Than 13 Other Host Receptors Besides ACE2!
Source: COVID-19 News Sep 24, 2021 3 years, 7 months, 2 days, 4 hours, 23 minutes ago
COVID-19 News: A new study by researchers from the Genos Glycoscience Research Laboratory-Croatia and the University of Zagreb-Croatia has shockingly found that the SARS-CoV-2 coronavirus is able to not just bind to the ACE2 receptors but also are able to bind to 13 other identified human host receptors.
These host receptors include:
1. C-type lectin receptors
2. Mannose-binding lectin (MBL)
3. DC-SIGN including
4. L-SIGN
5. Macrophage galactose type C-type lectin (MGL)
6. Mannose receptor
7. Lectin-like oxidized low-density lipoprotein receptor-1
8. Liver and lymph node sinusoidal endothelial cell C-type lectin
9. Toll-like receptors (TLRs)
10. Neuropilin-1
11. Glucose-regulated protein 78 (GRP78)
12. Siglecs
13. Galectins
Some of these receptors were identified as potential receptors in early studies but were never followed up.
Some other scientist who were not involved in the study speculate that even more so, the emerging SARS-CoV-2 variants with newer mutations on their spike proteins are adapting such that they are able to not only bind to other human host receptors but also evade human immune responses or even disrupt and shut down human immune responses for a while.
Some of the possible consequences of that is that we will see more people initially being infected expressing either asymptomatic or simply mild symptomatic manifestations while the SARS-CoV-2 could be doing more damage to the human cellular pathways, tissues and organs through newer pathogenesis modes, resulting in more chronic long term medical conditions!
This is something that needs to be studied more as while the world is in a stage of opening up and loosening COVID-19 restrictions etc and many assuming that the disease will become endemic and everybody is taking things lightly while the world might be having a catastrophic health crisis looming with Long COVID or PASC.
According to the study team, “The SARS-CoV-2 coronavirus infection displays a wide array of clinical manifestations. Although some risk factors for COVID-19 severity and outcomes have been identified the underlying biologic mechanisms are still not well understood. The surface SARS-CoV-2 proteins are heavily glycosylated enabling host cell interaction and viral entry. Angiotensin-converting enzyme 2 (ACE2) has been identified to be the main host cell receptor enabling SARS-CoV-2 cell entry after interaction with its S glycoprotein. However, recent studies report SARS-CoV-2 S glycoprotein interaction with other cell receptors, mainly C-type lectins which recognize specific glycan epitopes facilitating SARS-CoV-2 entry to susceptible cells. “
The study findings revealed that SARS-CoV-2 was able to interact and bind to other cell membrane surface receptors and soluble lectins, infleunzing viral cell entry, modulating its infectivity and potentially playing a role in subsequent clinical manifestations of COVID-19.
The study findings were published in the peer reviewed journal: Glycoconjugate Journal by Springer.
https://link.springer.com/article/10.1007/s10719-021-10021-z
The sSARS-CoV-2 coronavirus&rs
quo;s Spike (S) protein is partially responsible for the virus' pathogenicity. It is well known that the receptor-binding domain (RBD) of the S1 subunit of the S protein can bind to the angiotensin-converting enzyme 2 (ACE2) receptors, thus allowing viral entry into the host cell. Comparatively, the N terminal domain of the S2 subunit is responsible for membrane fusion.
However recent reports have shown that the S glycoprotein is capable of interacting with multiple alternate cell receptors.
The study team from the Genos Glycoscience Research Laboratory in Croatia had recently investigated the sites that the S protein is capable of binding with.
It is known that each protomer of the S glycoprotein contains 22 N-glycosylation sites and three O-glycosylation sites.
Past studies however show inconsistent results when characterizing glycosylation, with some researchers showing full N-glycosylation the majority of the time, and others only partial glycosylation.
https://academic.oup.com/glycob/article/30/12/981/5826952
https://www.biorxiv.org/content/10.1101/2020.07.29.227462v1
Furthermore there has also been disagreement about the type of glycans found, with alternate studies showing hybrids, high-mannose, and complex N-glycans.
This wide variation may be due to the method by which the S glycoprotein is glycosylated, as it uses the hosts’ glycosylation mechanisms that ultimately result in patterns similar to the host cells. This would result in different glycosylation patterns and different types of glycans, depending on the type of cell that the particular virus was constructed in.
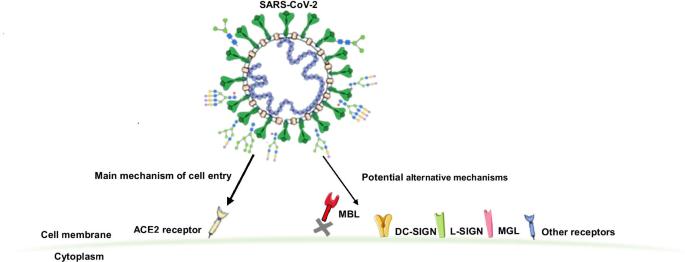
It has also been known that the binding of the SARS-CoV-2 RBD to ACE2 is facilitated by the glycosaminoglycan heparan sulfate interaction of cellular glycocalyx, which aids the S protein structure to a more open conformation. However, while ACE2 is the primary receptor for SARS-CoV-2 cell entry, it shows low expression in the respiratory system.
https://pubmed.ncbi.nlm.nih.gov/32970989/
https://www.embopress.org/doi/full/10.15252/msb.20209610
https://jamanetwork.com/journals/jama/fullarticle/2766524
https://www.biorxiv.org/content/10.1101/2020.05.29.123513v1
Considering that SARS-CoV-2 primarily spreads between hosts through droplet infection, this indicates that the S glycoprotein can interact with other receptors in order to enter the cell.
Potent Nuclear Magnetic Resonance (NMR) spectroscopy revealed that N glycans of the RBD can bind to various lectins, including macrophage galactose lectin (MGL), galectins -3, -7, and -8, as well as sialic acid-binding immunoglobulin-type lectin. https://onlinelibrary.wiley.com/doi/10.1002/anie.202011015
Interestingly expression levels of MGL have been found to be elevated in patients with severe coronavirus disease 2019 (COVID-19). This observation potentially suggests that the virus could respond to immune reactions through cell entry via these receptors.
Potentially more alarming is the possibility of the S glycoprotein binding to C-type lectin receptors (CLRs). These can be bound by oligomannose N-glycans; in fact, approximately 1 out of every 3 SARS-CoV-2 S glycans are of this type.
CLRs are primarily found on antigen-presenting cells, such as macrophages and dendritic cells. Both of these cell types are often found in the lungs.
In fact, when inflammation occurs, monocytes circling in the blood and other tissues can also differentiate into these cells. This would provide both an initial route for infection in the respiratory system if ACE2 showed low expression, and for viral entry into cells in the bloodstream.
Furthermore macrophage galactose lectin (MGL) is another receptor primarily expressed in dendritic cells and macrophages in the human lungs and respiratory system. MGL recognizes glycans bearing terminal galactose.
Studies covered in various
COVID-19 News have already demonstrated that SARS-CoV-2 is able to bind to this lectin, further supporting the belief that the virus has alternate routes for cell entry. Further examination into the amino acid sequencing suggests that both N and O glycans are involved in MGL binding.
https://pubmed.ncbi.nlm.nih.gov/34015061/
The study team found that the reviewed studies show that the SARS-CoV-2 S glycoprotein can bind to multiple other receptors, both allowing trans-infection of susceptible cells, as well as allowing more types of cells to be infected. The trans-infection of cells will not only lead to greater viral transmission but as the types of cells vulnerable to trans-infection are mostly immune cells, this could exacerbate the risk of severe inflammation and cytokine release.
In addition, the glycosylation-mediated interactions with SARS-CoV-2 could suggest that the glycosylation of SARS-CoV-2 could have significant effects on the severity of the disease in the patient, to the extent where it could determine recovery or spread. This suggests a mechanism for asymptotic SARS-CoV-2 infection, as a decreased titer of SARS-CoV-2 due to endocytosis by immune cells in the lungs may prevent symptoms from appearing.
Further detailed urgent research is warranted as the implications of the study findings are worrisome in so many aspects.
For the latest
COVID-19 News, keep on logging to Thailand Medical News.

