BREAKTHROUGH! COVID-19 Vaccines: ImmunityBio Inc. And Icahn Develop Next Generation Bivalent hAd5 Vaccine That Elicits Rapid T-Cell And Antibody Response
Source: COVID-19 Vaccines Aug 03, 2020 4 years, 8 months, 1 week, 3 days, 12 hours, 27 minutes ago
COVID-19 Vaccines: American researchers from ImmunityBio Inc. and Icahn School of Medicine at Mount Sinai in New York have developed a next generation COVID-19 vaccine: the bivalent human adenovirus serotype 5 (hAd5) vaccine for inducing both cellular and humoral immunity against the SARS-CoV-2 coronavirus, using both an S protein sequence optimized for cell surface expression and a conserved nucleocapsid antigen designed to be transported to the endosomal subcellular compartment.
.jpg)
The breakthrough research findings were published on a preprint server and are currently being peer-reviewed.
https://www.biorxiv.org/content/10.1101/2020.07.29.227595v1
The SARS-CoV-2 coronavirus utilizes S protein and its receptor-binding domain (RBD) to interact with angiotensin-converting enzyme 2 (ACE2) to enter human cells. Consequently, most vaccines under development target S protein in order to elicit the production of antibodies against RBD, but also to adequately steer T cell immune response.
Despite the urgent need for rapid SARS-CoV-2 vaccine development, reliance on only one antigen cargo or immunological pathway (as currently seen in most of the monovalent vaccines under development) is not without risks. For example, new viral strains with mutations in S protein may emerge and render developed vaccines ineffective.
As such in order to overcome the aforementioned risk and provide additional antigens, the researchers from ImmunityBio Inc. and Icahn School of Medicine at Mount Sinai in New York have added an optimized N protein sequence, which has a role in viral replication, particle assembly, and release.
The new vaccine platform utilized in this study represents a next-generation recombinant human adenovirus serotype 5 (hAd5) vector that harbors deletions in the E1, E2b, and E3 gene regions.
This new vector can primarily be distinguished from other first-generation (E1-, E3-) recombinant Ad5 platforms by having additional deletions in the early gene 2b (E2b) region, which remove the expression of the viral DNA polymerase.
Please Help To Donate To Sustain This Site And Other Research We Are Propelling. Thank You. https://www.thailandmedical.news/p/sponsorship
As these deletions make the hAd5 platform efficacious even in the presence of existing adenovirus immunity, it can enable relatively long-term antigen expression without significant induction of anti-vector immunity. Even more important, this next generation Ad vector has shown to be safe in more than 125 patients with solid tumors.
Upon vector optimization, this new innovative vaccine construct design basically comprised an S-Fusion + N-ETSD sequence. More specifically, the researchers have first designed an enhanced T-cell Stimulation Domain (ETSD) to nucleocapsid antigen (N) to allow the necessary processing and presentation.
r />
Furthermore, the researchers have optimized the wild type S protein "S Fusion sequence" to display the highly antigenic RBD region of S protein on the cell surface. This was done to increase the stability and the likelihood of native folding.
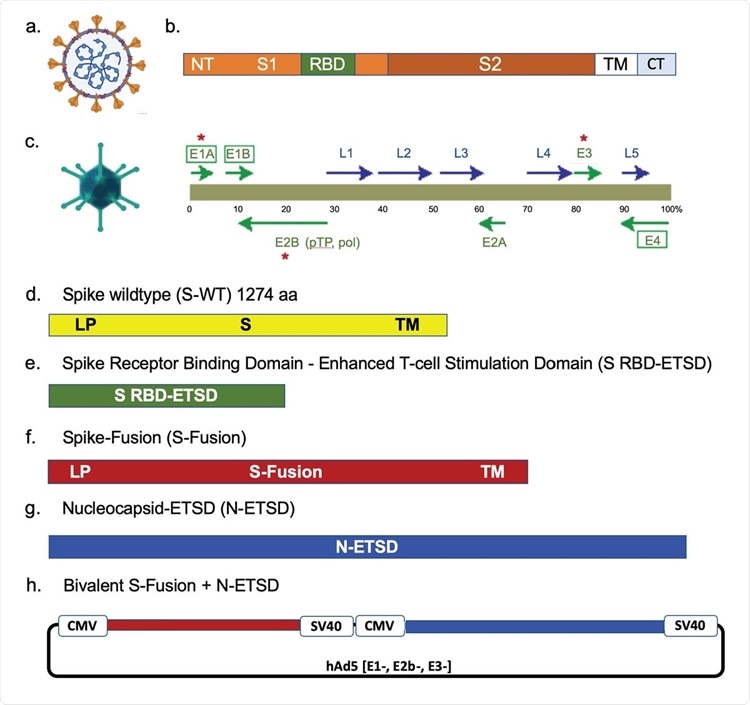
The SARS-CoV-2 virus, spike, the hAd5 [E1-, E2b-, E3-] vector and vaccine candidate constructs. (a) Trimeric spike (S) protein ( ▼) is displayed on the viral surface; the nucleocapsid (N) protein (⬤) is associated with the viral RNA. (b) The Receptor Binding Domain (RBD) is within the S1 region, followed by other functional regions, the transmembrane domain (TM) and the C-terminus (CT), which is within the virus. (c) The second-generation human adenovirus serotype 5 (hAd5) vector used has the E1, E2b, and E3 regions deleted. Constructs are shown for (d) S wild type (S-WT), (e) S-RBD with the Enhanced T-cell Stimulation Domain (S RBD-ETSD), (f) S-Fusion, (g) N-ETSD, and (h) bivalent hAd5 S-Fusion + N-ETSD; LP – Leader peptide.
Dr Patrick Soon Shiong, co-researcher from ImmunityBio Inc -California told Thailand Medical News, “This new next generation COVID-19 vaccine offers greater protection against SARS-CoV-2. A key finding of our construct is that N-ETSD, which we show is directed to the endosomal/lysosomal compartment, elicits a CD4+ response, a necessity for induction of memory T cells and helper cells for B cell antibody production."
The rapid T-cell response to both S and N antigens expressed by hAd5 S-Fusion + N-ETSD included the production of multiple cytokines, such as interferon-gamma and tumor necrosis factor-alpha, which is somewhat consistent with successful antimicrobial immunity against bacterial and viral infections.
Also such polycytokine T-cell responses to SARS-CoV-2 N protein are in line with recovered COVID-19 patients, indicating that the bivalent hAd5 S-Fusion + N-ETSD vaccine may provide vaccine subjects with greater protection against SARS-CoV-2.
In comparison to the N protein, the S protein (here expressed as S-Fusion with enhanced RBD cell-surface expression and conformational integrity) generated CD8+ T cells predominantly in this study. Finally, both the T-cell and antibody immune responses to S and N demonstrated a T-helper 1 (Th1) bias.
Dr Soon Shiong added, “The vaccine exhibited robust T and B cell response. Our results confirmed our vaccine design goal, showing that S-Fusion induced elevated levels of antigen-specific T-cell responses against S compared to wild-type spike protein.”
Significantly, the potency of the antibody response generated after vaccinating with hAd5 S-Fusion + N-ETSD revealed evidence of a high neutralization effect, which was achieved even at a high dilution factor.
Dr Mohit Verma, a co-researcher from ImmunityBio Inc -California further added, "Based on these findings, we are advancing this next generation bivalent hAd5 S-Fusion + N-ETSD vaccine as our lead clinical candidate to test for its ability to provide robust, durable cell-mediated and humoral immunity against SARS-CoV-2 infection.”
The research team is also currently conduction studies exploring this vaccine construct in oral, sublingual, and intranasal formulations to induce mucosal immunity (alongside cell-mediated and humoral immunity).
This research development is a major breakthrough in the quest to discover ideal COVID-19 Vaccines that will generate long-term T and B cell memory response.
Please Help To Donate To Sustain This Site And Other Research We Are Propelling. Thank You. https://www.thailandmedical.news/p/sponsorship
.jpg)
