Breakthrough German Study Discovers That SARS-CoV-2 VOCs Dependent On IFITM2 For Replication. Anti-IFITM2 Antibody Inhibits The VOCs Effectively!
Source: SARS-CoV-2 Research-IFITM2 Dec 17, 2021 3 years, 4 months, 1 week, 2 days, 4 hours, 16 minutes ago
SARS-CoV-2 Research: A new study by researchers from the Institute of Molecular Virology at Ulm University Medical Center-Germany and the Gene Center at LMU München-Germany has found that the various SARS-CoV-2 variants of concern or VOCs are highly dependent on Interferon-inducible transmembrane proteins or IFITMs for effective replication especially in iPSC-derived alveolar epithelial type II (iATII) cells, the target cells of SARS-CoV-2 in human lungs and effectively silencing IFITM2 using anti-IFITM2 antibodies inhibited the various SARS-CoV-2 VOCs.
Please Help! Do Not Ignore Our Appeals For Help. Please support the sustainability of this website and all our research and community initiatives by making a donation to our cause. Your donation helps saves lives directly and indirectly. Every dollar counts. Please Support. Thank You. (We apologize for the constant appeal.)
https://www.thailandmedical.news/p/sponsorship

The study findings were published on a preprint server and are currently being reviewed.
https://www.biorxiv.org/content/10.1101/2021.11.17.468942v2
The IFITM (interferon-induced transmembrane) proteins comprise a family of interferon-induced antiviral cell-intrinsic restriction factors with high constitutive expression in many cells, including barrier epithelial cells. As their names imply, the expression of human IFITM1, IFITM2, and IFITM3 is also strongly upregulated by both type I and type II interferons. Uniquely among known restriction factors, these proteins prevent viruses from traversing the lipid bilayer of the cell and accessing the cytoplasm. The IFITM proteins potently restrict a number of viral pathogens important to human health, such as dengue virus (DENV) and influenza A virus (IAV), but intriguingly have little or no effect on many other viruses for example, the arenavirus that causes Lassa fever (LASV).
IFITM proteins have a short N-terminal and C-terminal domain, two transmembrane domains (TM1 and TM2) and a short cytoplasmic domain. The first transmembrane domain (TM1) and the cytoplasmic domain are conserved among different IFITM proteins in humans and mice.In the absence of interferon stimulation, IFITM proteins can express broadly in tissues and cell lines. In humans, IFITM1, IFITM2 and IFITM3 are able to express in different tissues and cells while the expression of IFITM5 is limited to osteoblasts. The type I and II interferon induce IFITM proteins expression significantly. IFITM proteins are involved in the physiological process of immune response signaling, germ cell maturation and development.
The interferon-inducible transmembrane proteins (IFITMs), including IFITMs 1, 2, and 3, are well-known for their inhibitory effects against numerous viral pathogens including the highly pathogenic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As such it comes as a surprise to many that the SARS-CoV-2 coronavirus is able to use an antiviral component of the human immune system to assist itself in effective replication!
A previo
us study had shown that an early SARS-CoV-2 isolate (NL-02-2020) hijacks interferon-induced transmembrane proteins (IFITMs) for efficient replication in human cells.
https://www.nature.com/articles/s41467-021-24817-y
Since then, several Variants of Concern (VOCs) showing increased infectivity and resistance to neutralization have emerged and globally replaced the early viral strains.
The study team was determined to ascertain as to whether the four SARS-CoV-2 VOCs (Alpha, Beta, Gamma and Delta) maintained the dependency on IFITM proteins for efficient replication.
The study findings found that depletion of IFITM2 strongly reduces viral RNA production by all four VOCs in the human epithelial lung cancer cell line Calu-3.
Silencing of IFITM1 had little effect, while knock-down of IFITM3 resulted in an intermediate phenotype.
However, strikingly, depletion of IFITM2 generally reduced infectious virus production by more than four orders of magnitude. In addition, an antibody directed against the N-terminus of IFITM2 inhibited SARS-CoV-2 VOC replication in iPSC-derived alveolar epithelial type II cells thought to represent major viral target cells in the lung.
The study findings show that endogenously expressed IFITM proteins (especially IFITM2) are critical cofactors for efficient replication of genuine SARS-CoV-2 VOCs, including the currently dominating Delta variant.
The
SARS-CoV-2 Research team felt that as several SARS-CoV-2 variants of concern (VOCs) are emerging globally and replacing previous strains, it is crucial to investigate the role of IFITMs in VOC replication.
The study team demonstrated that the Alpha, Beta, Gamma, and Delta variants of SARS-CoV-2 require endogenous IFITM2 expression for efficient replication.
In addition, the study team found that an anti-IFITM2 antibody inhibits the replication of the Delta variant in iATII cells by more than 90%.
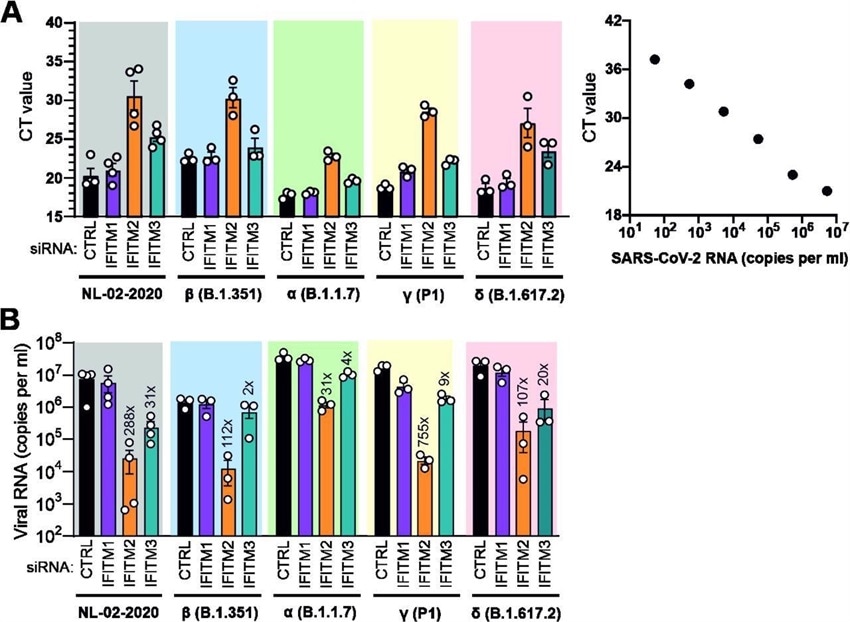 Role of IFITMs in replication of SARS-CoV-2 VOCs in Calu-3 cells. (A) Standard curve and raw qRT-PCR CT values obtained using supernatants of Calu-3 cells collected 2 days post-infection. (B) Viral N RNA levels in the supernatant of Calu-3 cells infected with the indicates SARS-CoV-2 variants. Cells were transfected with control (CTRL) or IFITM targeting siRNAs as indicated. Numbers above the bars indicate n-fold reduction compared to the viral RNA levels detected in the supernatant of Calu-3 cells treated with CTRL siRNA. Bars in panel A and B represent the mean of 3 to 4 independent experiments (±SEM) each measured in technical duplicates.
Study Schematics
Role of IFITMs in replication of SARS-CoV-2 VOCs in Calu-3 cells. (A) Standard curve and raw qRT-PCR CT values obtained using supernatants of Calu-3 cells collected 2 days post-infection. (B) Viral N RNA levels in the supernatant of Calu-3 cells infected with the indicates SARS-CoV-2 variants. Cells were transfected with control (CTRL) or IFITM targeting siRNAs as indicated. Numbers above the bars indicate n-fold reduction compared to the viral RNA levels detected in the supernatant of Calu-3 cells treated with CTRL siRNA. Bars in panel A and B represent the mean of 3 to 4 independent experiments (±SEM) each measured in technical duplicates.
Study Schematics
The study team performed full-genome sequence analyses of all the SARS-CoV-2 Alpha, Beta, Gamma, and Delta VOCs to determine whether they require IFITM proteins for efficient replication. The team also verified that these variants show expected mutations in spike (S) protein and compared all results to the NL-02-2020 isolate.
The study team next performed small interfering ribonucleic acid RNA (siRNA) knockdown (KD) studies in the human epithelial lung cancer cell line Calu-3, which endogenously expresses ACE2, and all three IFITM proteins to examine the role of endogenous IFITM expression on infection by SARS-CoV-2 VOCs.
All viral replication was examined by quantitative reverse transcription-polymerase chain reaction (qRT-PCR), which estimated viral nucleocapsid (N) RNA levels in the cell culture supernatants two days after infection with SARS-CoV-2 variants.
Research Findings
The study team made some interesting observations about the role of IFITMs in the replication of all four SARS-CoV-2 VOCs. While depletion of IFITM2 reduced infectious virus production by more than four orders of magnitude by all four VOCs in the Calu-3 cell line, silencing of IFITM1 expression had little or no effect.
Interestingly when compared to NL-02-2020, most VOCs produced 2- to 4-fold higher levels of viral RNA in Calu-3 cells, with the Beta variant exhibiting moderately lower levels of viral RNA.
Importantly when IFITM2 was silenced, the Alpha, Beta, and Gamma variants showed a 32-fold, 112-fold, and 754-fold decrease in viral RNA yields, respectively.
Most significantly, IFITM2 silencing reduced viral RNA yields by the Delta variant by more than 100-fold, whereas IFITM3 silencing was associated with a 20-fold reduction. Taken together, these observations demonstrate that the Delta variant requires IFITM proteins for efficient entry.
With the exception of the Beta variant, all SARS-CoV-2 variants produced more than 10 million infectious virus particles per milliliter (ml) culture supernatant in Calu-3 cells treated with the control siRNA. In striking contrast, infectious virus production was generally reduced to less than or equal to 100 infectious particles per ml culture supernatant upon silencing of IFITM2.
Although the iATII cells express IFITM2 and IFITM3 like Calu-3 cells, as indicated by Western blot analyses, both cell types show little or no detectable expression of IFITM1. Also, ACE2 expression in iATII cells was not detected by Western blot analyses, though it was readily detectable in Calu-3 cells.
Importantly an antibody directed against the N-terminus of IFITM2 completely inhibited the replication of VOCs in iATII cells. This reduction in viral RNA production was dose-dependent and occurred at varying efficiencies. The anti-IFITM2 antibody reduced the replication of the Delta variant in iATII cells by up to 95%.
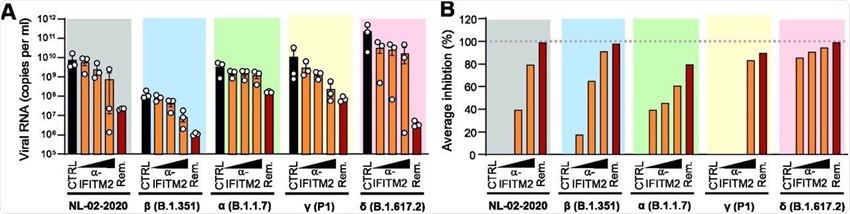 Effect of an α-IFITM2 antibody on replication of SARS-CoV-2 variants in iATII cells. (A) Quantification of viral N RNA levels in the supernatant of iATII cells treated with α-IFITM2 antibody (20, 40, or 80 µg/ml) or Remdesivir (10 µM) 1 h before infection (SARS-CoV-2, MOI 0.5), collected 48 h post-infection. Bars represent the mean of three independent experiments. (B) Average percentage of reduction of vRNA levels in the supernatants of (E) compared to the untreated control.
It was found that the Alpha and Delta variants contain a mutation that affects interferon sensitivity, proteolytic activation, and fusogenicity of the S protein.
As such, the Alpha variant yielded about 100-fold higher levels of viral RNA upon silencing IFITM2 expression as compared to the Beta variant.
Effect of an α-IFITM2 antibody on replication of SARS-CoV-2 variants in iATII cells. (A) Quantification of viral N RNA levels in the supernatant of iATII cells treated with α-IFITM2 antibody (20, 40, or 80 µg/ml) or Remdesivir (10 µM) 1 h before infection (SARS-CoV-2, MOI 0.5), collected 48 h post-infection. Bars represent the mean of three independent experiments. (B) Average percentage of reduction of vRNA levels in the supernatants of (E) compared to the untreated control.
It was found that the Alpha and Delta variants contain a mutation that affects interferon sensitivity, proteolytic activation, and fusogenicity of the S protein.
As such, the Alpha variant yielded about 100-fold higher levels of viral RNA upon silencing IFITM2 expression as compared to the Beta variant.
While on the average, the Delta variant replicated to about 30-fold higher levels than the early NL-02-2020 isolate in iATII cells, this indicates that the Alpha variant shows reduced susceptibility to IFN inhibition and is less dependent on IFITM2 for efficient infection as compared to other SARS-CoV-2 variants.
The study findings collectively show that IFITMs, especially IFITM2, are critical cofactors for the efficient replication of the tested SARS-CoV-2 VOCs, including the currently dominant Delta variant.
Significantly another evidence to support this finding is that an a-IFITM2 antibody inhibits the replication of the Delta variant in iATII cells by more than 90%.
Hence overall, the study findings suggest that IFITM2 plays a key role in SARS-CoV-2 transmission which, in turn, shows that IFITM2 may be a target for preventive or therapeutic interventions against SARS-CoV-2 since all VOCs, including the currently dominant Delta variant, are dependent on it for dissemination and pathogenesis.
It should be interesting to determine whether the emerging Omicron variant is also dependent on IFITM2 for efficient infection and susceptible to inhibition by a-ITITM2 antibodies. Furthermore, the results of the current study provide evidence for higher efficiency of the Delta variant to replicate in human lung cells as compared to the early SARS-CoV-2 isolate, thereby explaining why the Delta variant infects and transmits with higher efficiency as compared to the other VOCs.
The research findings showed that the silencing of IFITM2 expression had a higher effect on infectious titers than on viral RNA yields. Research studies in the future should focus on determining whether the presence of IFITM2 has an enhancing effect on the infectiousness of SARS-CoV-2 particles. These studies may also help to determine the exact mechanism of IFITM2-dependent enhancement or whether background levels are higher for viral RNA due to release from or lysis of infected cells. Future studies should focus on revealing the broad antiviral activity of IFITMs involving alterations in cellular membrane rigidity and curvature instead of specific interactions with viral glycoproteins.
The same study team is currently conduction further research on the Omicron variant and the study findings should be released in about a fortnights time.
Please Help! Do Not Ignore Our Appeals For Help. Please support the sustainability of this website and all our research and community initiatives by making a donation to our cause. Your donation helps saves lives directly and indirectly. Every dollar counts. Please Support. Thank You. (We apologize for the constant appeal.)
https://www.thailandmedical.news/p/sponsorship
For the latest
SARS-CoV-2 Research, keep on logging to Thailand Medical News.


 Effect of an α-IFITM2 antibody on replication of SARS-CoV-2 variants in iATII cells. (A) Quantification of viral N RNA levels in the supernatant of iATII cells treated with α-IFITM2 antibody (20, 40, or 80 µg/ml) or Remdesivir (10 µM) 1 h before infection (SARS-CoV-2, MOI 0.5), collected 48 h post-infection. Bars represent the mean of three independent experiments. (B) Average percentage of reduction of vRNA levels in the supernatants of (E) compared to the untreated control.
Effect of an α-IFITM2 antibody on replication of SARS-CoV-2 variants in iATII cells. (A) Quantification of viral N RNA levels in the supernatant of iATII cells treated with α-IFITM2 antibody (20, 40, or 80 µg/ml) or Remdesivir (10 µM) 1 h before infection (SARS-CoV-2, MOI 0.5), collected 48 h post-infection. Bars represent the mean of three independent experiments. (B) Average percentage of reduction of vRNA levels in the supernatants of (E) compared to the untreated control.