Breakthrough Lysosomal TRAP Technology Offers New Hope Against SARS-CoV-2 Variants
Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 24, 2024 1 year, 3 weeks, 5 days, 56 minutes ago
Medical News:
Revolutionary Breakthrough Against Viral Threats
A cutting-edge study from leading research institutions, including the State Key Laboratory of Biochemical Engineering at the Chinese Academy of Sciences, Tokyo University of Agriculture and Technology-Japan, and Peking University-China, has unveiled a novel antiviral strategy: the lysosomal “TRAP” or lysoTRAP. This groundbreaking approach could revolutionize how we combat viral infections like SARS-CoV-2 and influenza, especially as new variants continue to emerge.
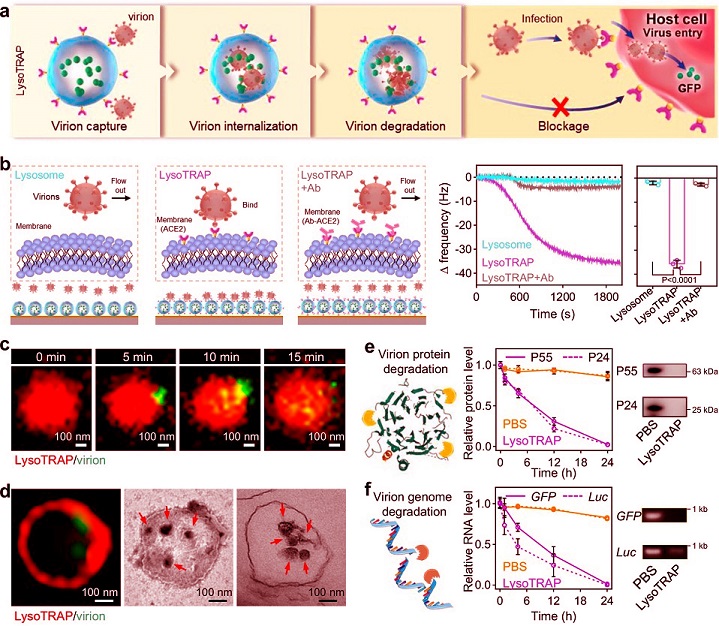 Evaluations of programmed performance of lysoTRAP in capturing, internalizing, and degrading pseudotyped SARS-CoV-2 virions, causing efficient inhibition of viral infection
a Schematic for showing the designed SARS-CoV-2 clearance mechanism by lysoTRAP. b Schematic illustration for quartz crystal microbalance (QCM) experiment. Corresponding QCM curves and the change of the frequency of each group were displayed on the right panels. c CLSM images of Cy5-labeled lysoTRAP co-incubated with BODIPY-labeled pseudotyped virions, showing the process of pseudotyped virion internalization by lysoTRAP. d STED image showing a high magnification of Cy5-labeled lysoTRAP with internalized BODIPY-labeled pseudotyped virions after a 4-hour co-incubation. Corresponding TEM imaging and TEM section observation were displayed on the right panels. The internalized pseudotyped virions were indicated by red arrows in lysoTRAP. e Enzyme-linked immunosorbent assay (ELISA) measurement of viral protein levels (P55 and P24: structural proteins in the pseudotyped virions) after lysoTRAP or PBS incubation for indicated time points. Corresponding western blotting (WB) results of the 24-hour incubated sample were displayed on the right panel. f Quantitative polymerase chain reaction (q-PCR) analysis of viral RNA levels (GFP and Luc) after incubating with lysoTRAP or PBS for indicated time points. Corresponding agarose gel electrophoresis results of the 24-hour incubated sample were displayed on the right panel.
Evaluations of programmed performance of lysoTRAP in capturing, internalizing, and degrading pseudotyped SARS-CoV-2 virions, causing efficient inhibition of viral infection
a Schematic for showing the designed SARS-CoV-2 clearance mechanism by lysoTRAP. b Schematic illustration for quartz crystal microbalance (QCM) experiment. Corresponding QCM curves and the change of the frequency of each group were displayed on the right panels. c CLSM images of Cy5-labeled lysoTRAP co-incubated with BODIPY-labeled pseudotyped virions, showing the process of pseudotyped virion internalization by lysoTRAP. d STED image showing a high magnification of Cy5-labeled lysoTRAP with internalized BODIPY-labeled pseudotyped virions after a 4-hour co-incubation. Corresponding TEM imaging and TEM section observation were displayed on the right panels. The internalized pseudotyped virions were indicated by red arrows in lysoTRAP. e Enzyme-linked immunosorbent assay (ELISA) measurement of viral protein levels (P55 and P24: structural proteins in the pseudotyped virions) after lysoTRAP or PBS incubation for indicated time points. Corresponding western blotting (WB) results of the 24-hour incubated sample were displayed on the right panel. f Quantitative polymerase chain reaction (q-PCR) analysis of viral RNA levels (GFP and Luc) after incubating with lysoTRAP or PBS for indicated time points. Corresponding agarose gel electrophoresis results of the 24-hour incubated sample were displayed on the right panel.
This
Medical News report delves into the remarkable mechanism behind lysoTRAP, a system that uses lysosomes - cellular structures known for degrading harmful substances - to target and destroy viruses. By anchoring virus-specific receptors, such as ACE2, onto lysosomes, researchers have created a tool that captures, internalizes, and degrades viruses, including resistant variants.
How LysoTRAP Works
LysoTRAP takes inspiration from the body’s immune system, particularly macrophages, which naturally capture and destroy pathogens in lysosomes. Researchers harvested lysosomes from activated macrophages, engineered them with a bait protein receptor - ACE2 for SARS-CoV-2 - and tested the resulting system against a range of viral strains.
Unlike conventional treatments, lysoTRAP targets the virus itself rather than its spike proteins, rendering it effective even against variants with mutations that evade existi
ng therapies. This adaptability was confirmed through extensive experiments using mouse models, hamster models, and human lung organoids.
The Study in Action
The team behind the study demonstrated that lysoTRAP not only captured but also internalized and degraded pseudotyped SARS-CoV-2 virions. A quartz crystal microbalance experiment showcased lysoTRAP’s high binding efficiency, while microscopy techniques confirmed that trapped viruses were effectively confined and degraded within the lysosome.
In vivo tests were equally promising. Mice administered lysoTRAP via pulmonary inhalation exhibited reduced viral presence in their lungs. Impressively, the system also worked against the Omicron variant, highlighting its resilience against mutations - a key challenge in combating COVID-19.
Extending LysoTRAP’s Potential
The researchers didn’t stop with SARS-CoV-2. By replacing the ACE2 receptor with a saccharide receptor, sialic acid (SA), they adapted lysoTRAP to target influenza viruses such as H1N1. The results were equally groundbreaking, with significant viral clearance observed in both cell culture and animal models.
This flexibility underscores lysoTRAP’s potential as a platform technology, paving the way for its adaptation against various viral pathogens. The study also highlighted the stability of lysoTRAP, which retains its functionality after long-term storage and freeze-drying.
Why This Matters
Current antiviral treatments often lose efficacy as viruses mutate. Vaccines and monoclonal antibodies, while effective initially, struggle against emerging variants. LysoTRAP offers a robust solution by focusing on the mechanisms that viruses use to invade host cells, bypassing reliance on specific viral proteins.
Moreover, its inhalable administration ensures direct delivery to the lungs, where respiratory viruses like SARS-CoV-2 and influenza typically take hold. This localized approach maximizes effectiveness while minimizing systemic side effects.
Key Findings
-Efficiency: LysoTRAP cleared SARS-CoV-2 and nine of its variants, including Omicron, in animal models and human lung organoids.
-Versatility: The platform was successfully adapted for H1N1 influenza, demonstrating broad applicability.
-Stability: LysoTRAP remained functional after six months of storage, showcasing its potential for real-world application.
-Safety: Tests revealed minimal inflammation or toxicity, even after repeated high-dose administrations.
Conclusions
The implications of this study are immense. By harnessing the natural degradation power of lysosomes and enhancing it with targeted receptors, lysoTRAP introduces a new paradigm in antiviral therapy. Its ability to adapt to different viruses and remain effective against variants makes it a game-changer in infectious disease management.
The researchers emphasize the potential for further refinements, such as optimizing lysosome production and exploring additional receptors for other viral families. These developments could extend lysoTRAP’s applications to a broader range of infectious diseases, offering hope in the face of ever-evolving viral threats.
As the study concludes, “The lysoTRAP platform holds promise as a universal antiviral strategy, capable of addressing current and future pandemics with unprecedented efficiency.”
The study findings were published in the peer-reviewed journal: Nature Communications.
https://link.springer.com/article/10.1038/s41467-024-54505-6
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/thymidine-phosphorylase-identified-as-key-driver-of-spike-protein-induced-blood-clots-in-covid-19
https://www.thailandmedical.news/news/new-insights-into-thyroid-dysfunction-triggered-by-covid-19-s-cytokine-storm
https://www.thailandmedical.news/news/covid-19-alters-drug-effects-through-cyp3a4-enzyme-dysregulation
