British Study Shows That Arthritis And Blood Cancer Patients Who Are On The Drug Rituximab Will Have Impaired Antibody Response To COVID Vaccines
Source: COVID-19 Vaccines-Rituximab Jan 30, 2022 3 years, 2 months, 2 weeks, 3 hours, 38 minutes ago
COVID-19 Vaccines-Rituximab: A new study conducted by researchers from the University of Birmingham-UK, University Hospital Birmingham NHS Foundation Trust-UK, The Royal Wolverhampton NHS Trust-UK and the Worcestershire Acute NHS Trust has found that arthritis and blood cancer patients who are on the drug rituximab will have impaired antibody response to all current COVID-19 vaccines.
It was found that most blood cancer and arthritis patients have a significantly reduced antibody response to double COVID-19 vaccination in the first six months of being treated with a widely used drug called rituximab.
B-cell-depleting agents like rituximab are among the most commonly used drugs to treat haemato-oncological and autoimmune diseases. These drugs rapidly induce a state of peripheral B-cell aplasia with the potential to interfere with nascent vaccine responses, particularly to novel antigens.
The study team examined the relationship between B-cell reconstitution and SARS-CoV-2 vaccine responses in two cohorts of patients previously exposed to B-cell-depleting agents: a cohort of patients treated for hematological B-cell malignancy and another treated for rheumatological disease.
The
COVID-19 Vaccines-Rituximab study found that B-cell depletion severely impairs vaccine responsiveness in the first 6 months after administration: SARS-CoV-2 antibody seroprevalence was 42.2% and 33.3% in the haemato-oncological patients and rheumatology patients, respectively and 22.7% in patients vaccinated while actively receiving anti-lymphoma chemotherapy. After the first 6 months, vaccine responsiveness significantly improved during early B-cell reconstitution; however, the kinetics of reconstitution was significantly faster in haemato-oncology patients.
The AstraZeneca ChAdOx1 nCoV-19 vaccine and the Pfizer BioNTech 162b vaccine induced equivalent vaccine responses; however, shorter intervals between vaccine doses (<1 m) improved the magnitude of the antibody response in haemato-oncology patients. In a subgroup of haemato-oncology patients, with historic exposure to B-cell-depleting agents (>36 m previously), vaccine non-responsiveness was independent of peripheral B-cell reconstitution.
The study findings have important implications for primary vaccination and booster vaccination strategies in individuals clinically vulnerable to SARS-CoV-2.
The study findings were published in the peer reviewed journal: Clinical & Experimental Immunology.
The study team aimed to establish whether the drug typically used to treat patients with haemato-oncological disease (e.g., certain blood cancers) or rheumatological disease (e.g., certain types of arthritis) ie Rituximab - may hinder the effectiveness of COVID-19 vaccines.
Typically, treatments such as Rituximab work by killing B cells, which are part of our immune system.
The research involved 80 patients receiving treatment for blood cancers and 36 being treated for diseases such as arthritis. Blood samples were taken before and after vaccination with two doses of either the Pfizer/BioNTech or Oxford/AstraZeneca COVID-19 vaccine between December 2020 and April 2021.
&nbs
p;
The results were then compared with a control group of healthy age-matched healthcare workers participating in other University of Birmingham-led COVID-19 studies.
The study findings showed that in the first six months following treatment with Rituximab, only 42.2% of patients with blood cancers and 33.3% of patients with arthritis developed an antibody response to double vaccination and this was reduced further to 22.7% in patients vaccinated while actively receiving anti-lymphoma chemotherapy.
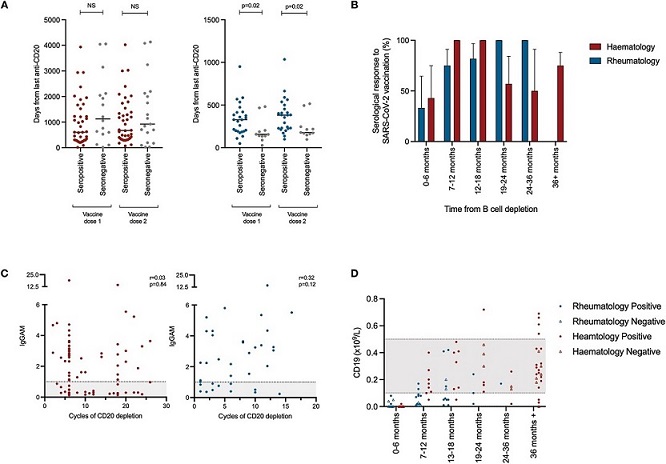 Immune reconstitution following CD20 depletion and vaccine responsiveness. (A) Time between last administration of anti-CD20 B-cell-depleting treatment and vaccine administration (left panel—haemato-oncology patients [red], right panel-rheumatology patients [blue]). (B) Seropositivity following SARS-CoV-2 vaccination with respect to time from last administration of anti-CD20 B-cell depletion. (C) Association between the magnitude of antibody responses and total prior exposure to anti-CD20 B-cell-depleting agents. IgGAM ratios less than 1.0 are considered negative and represented by the gray zone on the graph. (D) B-cell reconstitution and its association with vaccine responsiveness following treatment with anti-CD20 B-cell-depleting agents. Gray zones represent normal range for cellular population
Immune reconstitution following CD20 depletion and vaccine responsiveness. (A) Time between last administration of anti-CD20 B-cell-depleting treatment and vaccine administration (left panel—haemato-oncology patients [red], right panel-rheumatology patients [blue]). (B) Seropositivity following SARS-CoV-2 vaccination with respect to time from last administration of anti-CD20 B-cell depletion. (C) Association between the magnitude of antibody responses and total prior exposure to anti-CD20 B-cell-depleting agents. IgGAM ratios less than 1.0 are considered negative and represented by the gray zone on the graph. (D) B-cell reconstitution and its association with vaccine responsiveness following treatment with anti-CD20 B-cell-depleting agents. Gray zones represent normal range for cellular population
Furthermore, in those that did have an antibody response to vaccination, the strength of the response was much lower in those with blood cancer or arthritis compared to those in the healthy cohort.
The findings showed that six months after treatment, as patients' bodies began rebuilding B cells, vaccine responsiveness increased to 100% in blood cancer patients, while in arthritis patients there were also 'progressive increases' in overall vaccine responsiveness the greater the gap between vaccine and drug treatment.
The study findings showed similar quality and strength in antibody response with both types of COVID-19 vaccines and across both groups of patients. However, the data also show that shorter intervals (of less than a month) between the first and second vaccine significantly improved both quality and strength of antibody response in blood cancer patients.
Dr Supratik Basu, consultant hematologist at The Royal Wolverhampton NHS Trust and a professor at the University of Wolverhampton who also happens to be the corresponding author explained to Thailand
Medical News, "Rituximab targets B cells, which normally produce antibodies. Our hypothesis was that the drug might inadvertently affect the response to COVID-19 vaccination, which may leave patients very vulnerable to serious illness. These patients are already considered to be high-risk, and therefore it seemed sensible to determine whether the treatment for their pre-existing condition was stopping the body from producing the antibodies it needs to fight the virus. Here we could establish if further vaccination was required or if alternative protection was needed. As the rate of antibodies in this cohort was extremely low, this should alert clinicians to prioritize these patients for their third booster COVID-19 vaccine, while also giving important reminders about precautions such as hand-washing and social distancing."
Dr Adrian Shields, a clinical immunologist at the University of Birmingham and first author of the study added, "Rituximab is used all over the world to treat blood cancers and inflammatory diseases. We now know that its use is associated with poor responses to new vaccines. The results from our study emphasize how important it is to undertake further research to improve our understanding of how to use vaccines to achieve the best possible protection for our most clinically vulnerable patients."
Dr Mark Drayson, a professor of clinical immunodiagnostics at the University of Birmingham and Honorary Consultant Immunologist at University Hospital Birmingham and also co-corresponding author of the study, further added, "Our study provides important observations, which suggest that additional immunizations could be necessary at least six months after treatment with drugs that deplete B cells to optimize vaccine responsiveness. The monitoring of how a patient's immune response is rebuilding following treatment may also help to ensure optimal vaccine timing. We recommend that individuals who do not respond to COVID-19 vaccines should be evaluated further for secondary immunodeficiency, particularly if treatments that deplete B cells were last administered 18 months or more prior to vaccination."
For more about
COVID-19 Vaccines-Rituximab, keep on logging to Thailand Medical News.

