Causal Genes And Cell Types In The Genetic Blueprint Of Primary Open-Angle Glaucoma
Nikhil Prasad Fact checked by:Thailand Medical News Team Feb 17, 2024 1 year, 2 months, 1 week, 2 days, 19 hours, 7 minutes ago
Glaucoma News: Primary open-angle glaucoma (POAG) stands as the leading cause of irreversible blindness worldwide among individuals over the age of 55. Despite its prevalence, a cure remains elusive, and the intricacies of its biological mechanisms remain shrouded in mystery. Elevated intraocular pressure (IOP) has long been identified as a major risk factor, yet a significant portion of glaucoma patients exhibit normal eye pressure, raising questions about alternative pathogenic pathways.
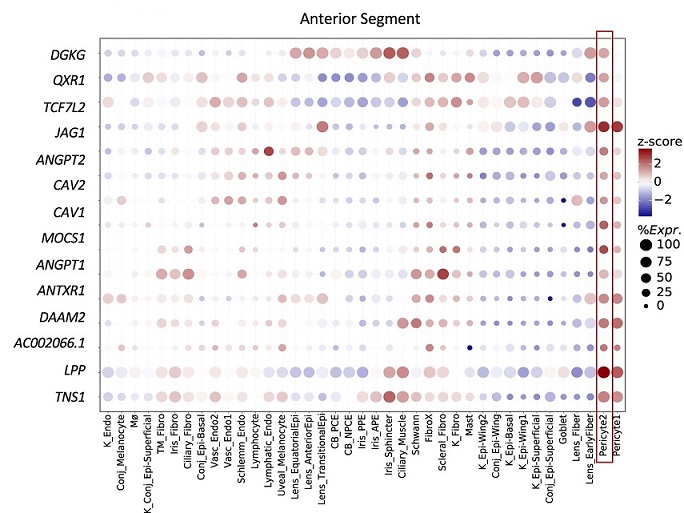 Bubble map displaying the expression of e/sGenes driving the IOP enrichment in pericytes (red box) across all cell types in the anterior segment. The colorbar represents gene expression z-scores computed by comparing each gene’s average expression in a given cell type to its average expression across all cell types divided by the standard deviation of all cell type expression averages. Bubble size is proportional to the percentage of cells expressing the given gene (log(TPK + 1) > 1).
A Breakthrough Comprehensive Study
Bubble map displaying the expression of e/sGenes driving the IOP enrichment in pericytes (red box) across all cell types in the anterior segment. The colorbar represents gene expression z-scores computed by comparing each gene’s average expression in a given cell type to its average expression across all cell types divided by the standard deviation of all cell type expression averages. Bubble size is proportional to the percentage of cells expressing the given gene (log(TPK + 1) > 1).
A Breakthrough Comprehensive Study
In a groundbreaking effort covered in this
Glaucoma News report, researchers from Massachusetts Eye and Ear, Department of Ophthalmology, Harvard Medical School-USA led by Dr Ayellet Segrè, Ph.D., spearheaded a study that merged genetic insights from a cross-ancestry genome-wide association study meta-analysis of POAG, led by Dr Janey Wiggs, MD, Ph.D., with a comprehensive meta-analysis of IOP, incorporating genetic regulation studies and single-cell expression measurements in glaucoma-relevant eye tissues.
Unlocking Genetic Mysteries
The integrated analyses unraveled hundreds of genes and regulatory effects associated with over 100 loci tied to POAG and/or IOP, shedding light on potential contributors to glaucoma risk through altered gene expression levels. These genes were found to be intricately linked with pathways crucial to disease mechanisms, including elastic fiber formation, extracellular matrix organization, vascular development, and neuronal-related processes.
The e/sQTL results imply that increased expression of CDKN2A, decreased expression of CDKN2B, and exon skipping in CDKN2B-AS1 may increase POAG risk. Other examples, involving a retina SLC2A12 eQTL overlapping a retinal CRE, and e/sQTLs acting on RERE and its antisense, RERE-AS1, that are physically linked via retina chromatin loops to the RERE transcription start site (TSS).
Cell Atlas Unveils Novel Insights
Leveraging single-nucleus gene expression data from a comprehensive cell atlas of the entire eye, the researchers identified various cell types susceptible to gene dysregulation, potentially influencing optic nerve degeneration. Fibroblasts in both conventional and unconventional outflow pathways, astrocytes in the retina and optic nerve head, oligodendrocytes, and vascular cells emerged as key players in the intricate web of POAG pathogenesis.
Potential Therapeutic Implications
The pathways uncovere
d by this extensive analysis have far-reaching implications for drug design targeting glaucoma. Notably, the findings suggest that focusing on neuronal support cells, in addition to retinal ganglion cells, could be crucial in designing novel drug and cell therapies. The research positions itself as a cornerstone for future endeavors aimed at deciphering the genetic regulation of gene expression in glaucoma-relevant eye tissues, ultimately offering a more comprehensive understanding of POAG risk and IOP variation.
Detailed Examination of Findings
The extensive investigation delved into the molecular and cellular causes of POAG across multiple loci associated with the condition. Elevated IOP, a known major risk factor, was scrutinized through a multi-ethnic GWAS meta-analysis, providing crucial insights into the genetic landscape of this complex trait.
Identification of Causal Genes
Through sophisticated colocalization and Mendelian randomization analyses, the researchers pinpointed putative causal genes for a significant portion of the GWAS loci associated with POAG and IOP. This approach proved vital in overcoming the challenges posed by noncoding variants and the intricate linkage disequilibrium inherent in these associations.
Unveiling Biological Processes
Integration of expression and splicing quantitative trait loci (e/sQTLs) data from diverse tissues provided a panoramic view of the genetic landscape implicated in glaucoma susceptibility. The enrichment of these loci in extracellular matrix organization, cell adhesion, and vascular development pathways emphasized the multifaceted nature of the biological processes involved in POAG.
Cell Type-Specific Enrichment
The application of cutting-edge methodologies such as ECLIPSER, a method designed to enrich causal loci and identify pathogenic cells, brought forth valuable insights into the cell types crucial for POAG development. Fibroblasts in outflow pathways, astrocytes, oligodendrocytes, and vascular cells emerged as pivotal contributors, signifying the complexity and diversity of cell types influencing optic nerve degeneration.
Novel Pathogenic Cell Types
Intriguingly, the study identified less-explored cell types such as pericytes, RPE cells, and various proliferative cells, adding layers of complexity to our understanding of POAG pathogenesis. These findings open avenues for further investigation into the roles of these cell types in glaucoma development and progression.
Insights into IOP-Dependent and Independent Mechanisms
By distinguishing between IOP-dependent and independent regulatory mechanisms, the research highlighted potential avenues for targeted therapeutic interventions. The identification of key processes like adherens junction regulation and cytoskeleton organization in IOP regulation broadens our understanding of the intricate mechanisms at play.
Implications for Neuroprotective Therapies
The identification of astrocytes and Müller glia cells in the retina as potential contributors to POAG emphasizes the need for a holistic approach to therapy. Rather than solely targeting retinal ganglion cells, the research suggests that neuroprotective therapies should also consider these support cells to combat glaucoma effectively.
Limitations and Future Directions
Despite the comprehensive nature of the study, certain limitations were acknowledged, including the potential absence of subthreshold associations and the necessity for further investigations into cellular heterogeneity. The researchers underscore the importance of future analyses, particularly those exploring the role of cellular heterogeneity within each cell type in POAG development.
Conclusion
In conclusion, this groundbreaking study has unraveled the intricate genetic and cellular tapestry of POAG, offering unprecedented insights into its pathogenesis. The identification of causal genes, elucidation of biological processes, and revelation of cell types influencing optic nerve degeneration present a roadmap for future therapeutic interventions. The research not only expands our understanding of POAG but also lays the foundation for targeted drug design and the development of neuroprotective therapies, marking a significant stride towards combating this prevalent cause of irreversible blindness.
The study findings were published in the peer reviewed journal: Nature Communications.
https://www.nature.com/articles/s41467-023-44380-y
For the latest
Glaucoma News, keep on logging to Thailand Medical News.
