Centriolar and Cytoskeletal Disruption by Viruses Including SARS-CoV-2 - A New Frontier in Viral Pathogenesis
Nikhil Prasad Fact checked by:Thailand Medical News Team Feb 07, 2025 2 months, 6 days, 2 hours, 19 minutes ago
Medical News: The Hidden Battlefield Within Our Cells
A new groundbreaking study has revealed how infectious pathogens, including viruses and intracellular parasites, manipulate the fundamental structures of our cells - the centrioles and cytoskeleton - to enhance their own survival and replication. Researchers from the Regional Centre for Biotechnology, Faridabad, India, in collaboration with other institutions, have uncovered the sophisticated strategies these pathogens use to hijack host cellular mechanisms.
 Centriolar and Cytoskeletal Disruption by Viruses Including SARS-CoV-2 - A New Frontier in Viral Pathogenesis
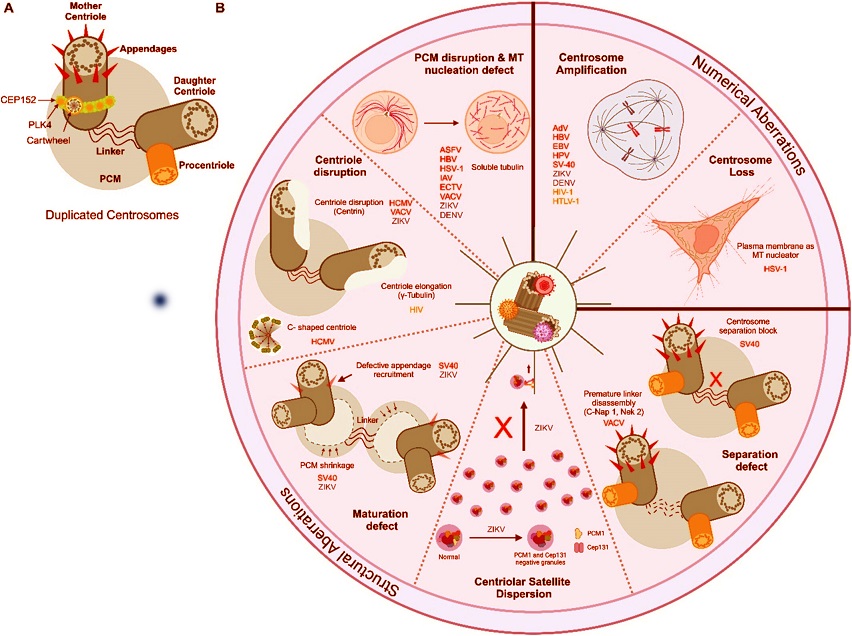
Virus-induced centriolar aberrations. (A) Schematic representing duplicating centrosomes with the key components. (B) Multiple structural aberrations are seen post-viral infection, characterized by PCM disruption, centriole disruption, centrosome maturation defect, centriolar satellite dispersion, and defects in centrosome duplication and separation. Numerical aberrations are characterized by either centrosome loss (acentriolar) or its amplification (supernumerary centrosomes). Viruses responsible for inducing the centriolar phenotype are highlighted individually as DNA viruses, RNA viruses, and Retroviruses.
Centriolar and Cytoskeletal Disruption by Viruses Including SARS-CoV-2 - A New Frontier in Viral Pathogenesis
Virus-induced centriolar aberrations. (A) Schematic representing duplicating centrosomes with the key components. (B) Multiple structural aberrations are seen post-viral infection, characterized by PCM disruption, centriole disruption, centrosome maturation defect, centriolar satellite dispersion, and defects in centrosome duplication and separation. Numerical aberrations are characterized by either centrosome loss (acentriolar) or its amplification (supernumerary centrosomes). Viruses responsible for inducing the centriolar phenotype are highlighted individually as DNA viruses, RNA viruses, and Retroviruses.
This
Medical News report delves into the findings of this extensive research, shedding light on how certain viruses, including SARS-CoV-2 and the Zika virus, target the delicate structural framework of cells, leading to severe consequences such as respiratory distress, neurological disorders, and impaired immune responses.
How Pathogens Exploit the Cellular Framework
The cytoskeleton, composed of actin filaments, microtubules, and intermediate filaments, is responsible for maintaining cell shape, enabling intracellular transport, and ensuring proper cell division. Many viruses and intracellular pathogens have evolved mechanisms to subvert this system, using it to their advantage. The study found that respiratory viruses, including influenza and SARS-CoV-2, directly interfere with microtubules and actin filaments to facilitate their movement within cells.
For instance, SARS-CoV-2 has been observed to cause severe ciliary dysfunction in airway cells, disrupting mucociliary clearance, a key defense mechanism of the respiratory system. Using micro-optical coherence tomography (μOCT), researchers identified a significant reduction in functional cilia, abnormal ciliary activity, and decreased ciliary beat frequency in infected individuals. Moreover, one of the viral proteins, ORF10, was found to promote the degradation of IFT46, a protein essential for cilium biogenesis. The impairment of this crucial cellular structure facilitates viral spread while compromising the body’s ability to clear pathogens from the respiratory tract.
Viral Hijacking of the Centriolar System
Beyond targeting the cytoskeleton, the study highlights how pathogens manipulate centrioles, the microtubule-organizing centers of ce
lls. The Zika virus, known for causing severe birth defects such as microcephaly, was shown to interfere with centrosome functions. Researchers identified that Zika viral proteins disrupt the recruitment of key centrosomal proteins such as Cep152, PCNT, and CPAP, leading to abnormal spindle formation and chromosome missegregation in neural stem cells. This disruption ultimately results in impaired brain development in infants infected in utero.
Similarly, other viruses, including herpes simplex virus (HSV-1) and Epstein-Barr virus (EBV), were found to downregulate FOXJ1 expression, a key regulator of multiciliated cell differentiation. This suggests that various viruses may share a common strategy of targeting the centriolar system to weaken the body’s cellular integrity.
The Role of the Cytoskeleton in Viral Replication
The research also revealed that viruses take advantage of cytoskeletal rearrangements to enhance their replication. Some viruses exploit actin-based structures known as “actin comets” to facilitate their entry into host cells, while others use microtubules to navigate through the dense cytoplasm. The study found that viral particles preferentially utilize post-translationally modified microtubules for efficient intracellular transport.
For instance, the influenza A virus (IAV) increases microtubule acetylation, which enhances viral trafficking and assembly at the plasma membrane. Additionally, the study found that some viruses promote hyperacetylation of microtubules to evade immune responses. The Epstein-Barr virus (EBV), for example, was shown to manipulate tubulin acetylation to prevent mitochondrial clearance, thereby enhancing its persistence in host cells.
The Immune System’s Struggle Against Cytoskeletal Manipulation
The study further suggests that the hijacking of the cytoskeleton by viruses may contribute to the severity of infections. By manipulating cellular structures, viruses not only facilitate their own replication but also suppress immune responses. One key finding was that the SARS-CoV-2 viral protein NS3 suppresses the innate immune response by targeting centrosomal components, leading to a weakened antiviral defense mechanism.
Moreover, the study found that loss of centrosomal integrity due to viral infections results in delayed cell cycle progression, increased genomic instability, and aberrant cell division. This could have implications for the development of cancer and other chronic diseases linked to persistent viral infections.
Potential Strategies for Therapeutic Intervention
Given the critical role that the cytoskeleton plays in viral infections, researchers are now exploring potential therapeutic interventions targeting these pathways. The study suggests that drugs designed to stabilize microtubules or prevent viral manipulation of centrosomal components could serve as effective antiviral therapies.
For example, inhibitors of histone deacetylase 6 (HDAC6), which regulate microtubule acetylation, were found to counteract the effects of viral hijacking. Additionally, small molecules that disrupt virus-cytoskeleton interactions are being tested for their ability to reduce viral replication and spread. These approaches could open new avenues for the treatment of viral infections that currently lack effective therapies.
Conclusion
This extensive research underscores the intricate ways in which viruses and intracellular pathogens exploit the host cytoskeleton and centriolar system to their advantage. By disrupting cellular structures, these pathogens not only enhance their replication but also weaken the body's immune defenses. The findings provide valuable insights into the underlying mechanisms of viral pathogenesis and highlight potential targets for therapeutic intervention.
Future research will need to delve deeper into the molecular interactions between viruses and the cytoskeleton, as well as investigate potential strategies to counteract these manipulations. Scientists emphasize that understanding these interactions is crucial for developing more effective antiviral treatments and preventing severe complications arising from infections.
The study findings were published in the peer-reviewed journal: Cytoskeleton.
https://onlinelibrary.wiley.com/doi/10.1002/cm.22004
For the latest on Viruses, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/omicron-variants-show-enhanced-binding-to-human-cells-and-actin
https://www.thailandmedical.news/news/breaking-news-scientists-discover-that-spike-protein-of-omicron-and-its-sub-lineages-binds-with-actin
https://www.thailandmedical.news/news/interleukin-1-beta-inhibits-sars-cov-2-spread-by-preventing-cell-fusion-through-actin-bundle
