Clinical Report By Abu Dhabi Doctors Published In JAMA Opthalmology Journal Reveals Ocular Adverse Events With China’s Sinopharm COVID-19 Vaccines!
Source: China's COVID-19 Vaccines Sep 05, 2021 4 years, 5 months, 4 days, 3 hours, 29 minutes ago
Doctors and medical researchers from the Eye Institute, Cleveland Clinic Abu Dhabi-UAE have reported instances of individuals experiencing various adverse ocular events as a result of having taken the
China’s Sinopharm COVID-19 vaccine.
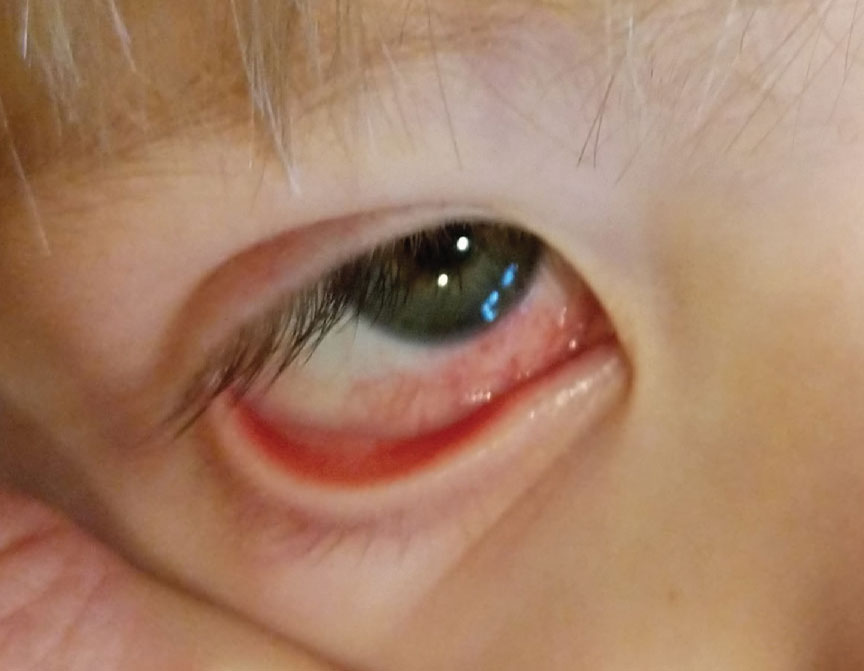
A total of 9 patients presented with ocular complaints 5.2 days after administration of the inactivated COVID-19 vaccine by Sinopharm.
The clinical case reports were published in the peer reviewed journal: JAMA Ophthalmology.
https://jamanetwork.com/journals/jamaophthalmology/fullarticle/2783653
One patient was diagnosed with episcleritis, 2 with anterior scleritis, 2 with acute macular neuroretinopathy, 1 with paracentral acute middle maculopathy, and 1 with subretinal fluid.
Episcleritis is an inflammatory condition affecting the episcleral tissue between the conjunctiva (the clear mucous membrane lining the inner eyelids and sclera) and the sclera (the white part of the eye).
Scleritis, or inflammation of the sclera, can present as a painful red eye with or without vision loss. The most common form, anterior scleritis, is defined as scleral inflammation anterior to the extraocular recti muscles.
Acute Macular Neuroretinopathy (AMN) is a rare disease first reported in 1975 (by Bos and Deutman). It is characterized by the sudden-onset of one or more paracentral scotomas. These scotomas generally persist indefinitely, though some resolve partially over months.
Paracentral acute middle maculopathy (PAMM) is a recently identified spectral-domain optical coherence tomography (SD-OCT) finding characterized by a hyper-reflective band spanning the inner nuclear layer (INL), which typically evolves to INL atrophy in later stages.
Subretinal fluid corresponds to the accumulation of a clear or lipid-rich exudate (serous fluid) in the subretinal space, i.e., between the neurosensory retina (NSR) and the underlying retinal pigment epithelium (RPE), in the absence of retinal breaks, tears, or traction
The study team warned that as vaccinations against COVID-19 continue, potential ocular adverse events should be reported in detail to increase awareness among the medical community, although typically, a causal relationship cannot be established definitively.
The clinical documentation of these adverse events took place from September 2020 to January 2021 at Cleveland Clinic Abu Dhabi, a tertiary referral center. Patients who reported ocular adverse events and presented within 15 days from the first of 2 doses of an inactivated Sinopharm COVID-19 vaccine from China were analyzed.
All of these affected patients underwent Snellen best-corrected visual acuity that was then converted to logMAR, applanation tonometry, and biomicroscopic examination with indirect ophthalmoscopy. Color fundus photography was obtained with a conventional 9-field fundus photography camera or with a widefield fundus photography system. Optical coherence tomography and optical coherence tomographic angiography images were obtained. Sex, race, age, and clinical data were self-reported.
According to the study team
, nine eyes of 7 patients (3
male individuals) presenting with ocular complaints following
China’s COVID-19 vaccines were included in the study. The mean (SD) age was 41.4 (9.3) years (range, 30-55 years); the mean best-corrected visual acuity was 0.23 logMAR (range, 0-1 logMAR; approximate Snellen equivalent, 20/32). The mean time of ocular adverse event manifestations was 5.2 days (range, 1-10 days).
At a mean of 6 days after the first inoculation, 2 of 9 eyes in the present series presented with acute unilateral vision loss associated with AMN and 1 of 9 eyes with PAMM. PAMM and AMN have been reported after H1N1 vaccination.
It should be noted that a previous study reported 2 patients with new paracentral scotoma secondary to AMN and PAMM 16 days after confirmed COVID-19 infections.
https://pubmed.ncbi.nlm.nih.gov/32620843/
One patient presented with bilateral, shallow areas of subretinal fluid with no thickening of the underlying choroid and an associated hypertrophy of the photoreceptor overlying the fluid. This picture was suggestive of a forme fruste of central serous chorioretinopathy that has previously been documented after smallpox and anthrax vaccination.
https://bjo.bmj.com/content/33/6/358
https://journals.lww.com/retinajournal/Citation/2004/08000/CENTRAL_SEROUS_CHORIORETINOPATHY_ASSOCIATED_WITH.23.aspx
Scleritis and episcleritis was reported in 4 of 9 cases at a mean of 5 days after the first dose of the vaccine. Although uncommon, there are few reports of episcleritis and scleritis following administration of live, attenuated viruses.
https://pubmed.ncbi.nlm.nih.gov/30222658/
Consistent with the reported literature, the present cases of scleritis noted soon after vaccination were mild.
It should also be noted that a previous study also reported a patient with episcleritis 7 days after confirmation of COVID-19 infection.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7300696/
The theoretic pathogenesis of an inactivated COVID-19–associated ocular inflammation is not known. Commonly proposed mechanisms have included both molecular mimicry and antigen-specific cell and antibody-mediated hypersensitivity reactions.
The study team said that in this case series study of 7 patients, the timing of transient and ocular complications 5.2 days after vaccination with an inactivated COVID-19 vaccine supported an association with the ocular findings, but a causal relationship cannot be established from this study design. As the urge for a vaccine against COVID-19 continues, the study team expects to see an increasing number of ocular adverse events from the various candidates.
For more about
China’s COVID-19 Vaccines, keep on logging to Thailand Medical News.
