Compared To Earlier SARS-CoV-2 Variants, Omicron Causes A Greater Degree Of Dysbiosis Of The Intestinal Microbiota!
Nikhil Prasad Fact checked by:Thailand Medical News Team Mar 06, 2024 1 year, 10 months, 2 weeks, 6 days, 40 minutes ago
COVID-19 News: As the COVID-19 pandemic evolves, researchers are continuously unraveling the intricate ways in which the SARS-CoV-2 virus interacts with the human body. Recent studies covered in this
COVID-19 News report, conducted by Sapporo Medical University School of Medicine-Japan, Shiga University of Medical Science, and Teine-Keijinkai Hospital in Sapporo-Japan have shed light on a fascinating aspect of the infection - its impact on the gut microbiota and metabolites.
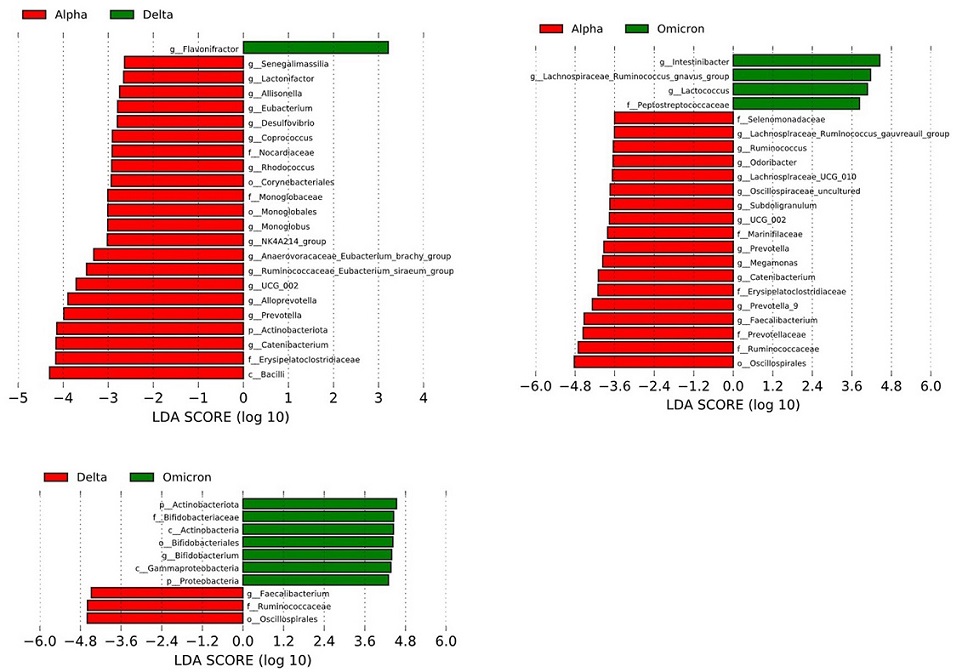 Fecal microbiome analysis comparing each infected variants in patients with COVID-19.
Enriched gut microbiota constituents were identified using the linear discriminant analysis (LDA) effect size (LEfSe). The histogram of the LDA scores with (log 10) values >3 and p < 0.05 revealed the most differentially abundant taxa among the different reproductive stages.
The Gastrointestinal Connection
Fecal microbiome analysis comparing each infected variants in patients with COVID-19.
Enriched gut microbiota constituents were identified using the linear discriminant analysis (LDA) effect size (LEfSe). The histogram of the LDA scores with (log 10) values >3 and p < 0.05 revealed the most differentially abundant taxa among the different reproductive stages.
The Gastrointestinal Connection
Patients with COVID-19 often present with gastrointestinal symptoms, including abdominal pain, diarrhea, nausea, vomiting, and anorexia. These symptoms have been linked to the virus's ability to infect human cells in the gastrointestinal tract through the angiotensin-converting enzyme 2 (ACE2)-mediated pathway. ACE2, highly expressed in intestinal epithelial cells, regulates the gut microbiota by influencing antimicrobial peptides in the small intestine.
Studies have previously shown that COVID-19 patients experience altered gut microbiota, with more severe cases exhibiting pronounced dysbiosis. Notably, patients with severe illness showed a marked decrease in ACE2 gene expression in the small intestine, leading to impaired tryptophan metabolism. Additionally, a decrease in short-chain fatty acids, such as butyric acid, has been observed in the intestinal metabolites of COVID-19 patients.
The SARS-CoV-2 Variant Differentiation
In the pursuit of understanding the nuances of gut microbiota alterations in COVID-19 patients, the researchers conducted a multicenter observational study involving 21 patients. The patients were stratified based on the SARS-CoV-2 variant they carried - six with the Alpha variant, 10 with the Delta variant, and five with the Omicron variant.
Fecal microbiome analysis revealed a distinct pattern: the α-diversity decreased in the order of Omicron, Delta, and Alpha variants. Linear discriminant analysis exposed variations in the abundance of short-chain fatty acid-producing gut microbiota for each SARS-CoV-2 variant. Notably, fecal metabolome analysis unveiled that both the Omicron and Delta variants exhibited significantly reduced levels of propionic and lactic acids compared to the Alpha strain.
Diving Deeper into the Data
To delve further into the study, the researchers analyzed fecal microbiome data for each SARS-CoV-2 variant, classifying patients into critical and non-critical groups. Surprisingly,
in the non-critical group, patients infected with the Omicron strain showed a substantial reduction in α-diversity compared to those with the Alpha strain. This observation suggested a clear alteration in the microbiota of non-critical Omicron-infected patients compared to their Alpha-infected counterparts.
Fecal metabolome analysis, focusing on short-chain fatty acids, underscored the impact of SARS-CoV-2 variants. Comparisons with healthy controls revealed lower levels of lactic and propionic acids in COVID-19 patients, with statistically significant differences observed in the Omicron strain. Further examination of the SARS-CoV-2 strains indicated marked decreases in lactic and propionic acids in the Delta and Omicron variants compared to the Alpha variant.
Fecal calprotectin analysis, a marker of intestinal inflammation, was also conducted. While there were no significant differences in calprotectin levels among the Alpha, Delta, and Omicron variants, an interesting finding emerged when comparing patients with and without gastrointestinal symptoms. Fecal calprotectin levels were notably higher in the non-critical group of Omicron-infected patients than in those with the Alpha and Delta variants.
Discussion and Implications
This groundbreaking study marks the first comparative analysis of gut microbiota and metabolites among patients infected with three different SARS-CoV-2 variants. The observed decrease in α-diversity, particularly in the Omicron variant, highlights the intricate relationship between SARS-CoV-2 variants and the gut microbiota.
The study also revealed a significant reduction in short-chain fatty acids, specifically lactic and propionic acids, in the stool metabolites of patients infected with the Omicron and Delta strains compared to the Alpha strain. Short-chain fatty acids play a crucial role in regulating the immune response in the intestinal tract, and their decrease could potentially induce intestinal inflammation.
The link between SARS-CoV-2 variants, dysbiosis, and intestinal inflammation raises important questions about the potential clinical implications of these findings. The Omicron variant, in particular, appears to be associated with altered gut microbiota and increased fecal calprotectin levels, even in non-critical cases. This suggests that the impact of SARS-CoV-2 variants on the gastrointestinal tract extends beyond the respiratory symptoms commonly associated with COVID-19.
Limitations and Future Directions
While this study provides valuable insights, it is not without limitations. The small sample size and the lack of a comprehensive classification of patients by disease severity limit the scope of statistical analysis and result interpretation.
Additionally, the study focused exclusively on Japanese patients, preventing cross-racial comparisons.
Future research endeavors should address these limitations by conducting larger-scale studies that include diverse populations and more comprehensive classifications based on disease severity. Exploring the timing and type of vaccination in relation to the gut microbiota could further enhance our understanding of the interplay between viral variants, host response, and vaccination.
Conclusion
In conclusion, this study unveils a novel dimension of the SARS-CoV-2 infection - its impact on the gut microbiota and metabolites. The findings highlight the distinctive alterations in gut microbiota composition and the reduction of short-chain fatty acids associated with different SARS-CoV-2 variants, particularly the Omicron strain. As the virus continues to mutate, understanding its effects on the gastrointestinal tract becomes increasingly crucial for comprehensive patient care and effective public health strategies.
The study's implications extend beyond the realm of COVID-19, emphasizing the intricate interplay between viral infections, the gut microbiome, and overall health. Further investigations into the mechanisms underlying these observations will pave the way for targeted therapeutic interventions and preventive strategies against the evolving landscape of viral infections.
The study findings were published in the peer reviewed journal: Frontiers in Microbiology.
https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2024.1358530/full
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
