Conformational Changes in COVID-19 Spike Protein Offer New Pathways to Block Viral Entry
NIkhil Prasad Fact checked by:Thailand Medical News Team Oct 28, 2024 5 months, 2 weeks, 2 days, 1 hour, 39 minutes ago
Medical News: A groundbreaking study has revealed a potential new approach to combatting COVID-19 by focusing on a particular protein structure in the SARS-CoV-2 virus. Researchers from Aalto University (Finland), Simon Fraser University (Canada), the Centre for Advanced Technologies (Uzbekistan), National Research University TIIAME (Uzbekistan), Loughborough University (UK), and the University of Antwerp (Belgium) investigated how certain molecular changes in the virus's structure might offer an innovative way to prevent it from entering human cells. By examining the Spike protein, an essential component for the virus’s infectivity, they identified that chemical alterations in specific regions of this protein could effectively block the virus from binding to host cells, thereby impeding the infection process.
 Conformational Changes in COVID-19 Spike Protein Offer New Pathways to Block Viral Entry
The Spike Protein: A Key to Viral Infection
Conformational Changes in COVID-19 Spike Protein Offer New Pathways to Block Viral Entry
The Spike Protein: A Key to Viral Infection
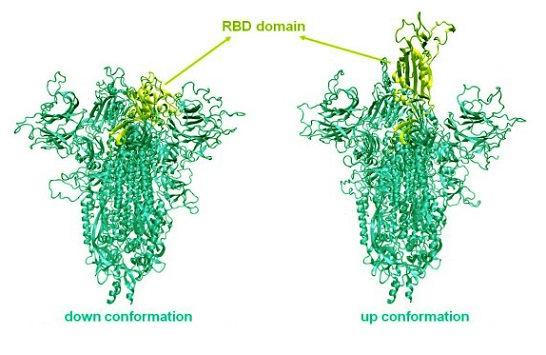
The COVID-19 pandemic is caused by the SARS-CoV-2 virus, which infects human cells through its Spike protein. This protein, which resembles a crown or “spike” on the surface of the virus, is essential for the virus’s ability to attach to human cells and initiate infection. It operates in two key positions: a "down" state, which hides it from immune defenses, and an "up" state, in which it is open and primed to bind to the ACE2 receptors on human cells. This receptor binding is what ultimately allows the virus to enter and infect cells.
A unique aspect of the Spike protein is its flexibility, which lets it switch between these states. When it shifts to the “up” state, the receptor-binding domain (RBD) is exposed and ready to bind to human cells, triggering infection. However, this study proposes that certain chemical modifications to the Spike protein could prevent these conformational changes, potentially locking the protein in a configuration that is less infective.
Focus on Cysteine: Key to Stabilizing the Spike Protein
In this
Medical News report, we delve into how researchers discovered that oxidizing specific cysteine residues in the Spike protein could significantly alter its behavior. Cysteine is a sulfur-containing amino acid found within proteins, and it plays a crucial role in maintaining protein structure due to its ability to form strong bonds with other cysteine molecules. When cysteine residues are oxidized, their bonding behavior changes, affecting the overall structure and stability of the protein.
The study team used molecular dynamics simulations, a type of computer modeling that allows scientists to observe and predict how molecules behave at the atomic level. By simulating the oxidation of cysteine residues in the Spike protein, they discovered that the energy required for the Spike protein to transition from the "down" state to the "up" state increased significantly.
Specifically, they found that oxidizing these cysteine residues raised the energy needed
to move from the down to up state by approximately 131 kJ/mol and from the up to down state by about 223 kJ/mol. These substantial energy increases suggest that oxidizing cysteine makes it much more challenging for the Spike protein to achieve the configurations needed for cell binding.
The increased energy requirement essentially means that the Spike protein is less likely to switch into the “up” conformation, the one needed for it to infect cells. By stabilizing the protein in the down position, where it is hidden and inactive, these modifications could act as a roadblock, preventing the virus from efficiently entering cells.
Salt Bridges and Hydrogen Bonds: Adding Stability to the Spike Protein
The Spike protein’s ability to switch between states is also influenced by interactions within the protein itself. Hydrogen bonds and salt bridges (electrostatic attractions between charged parts of the molecule) play a critical role in holding the protein’s shape and enabling its movement. The research showed that when cysteine residues are oxidized, new salt bridges form, increasing the rigidity of the Spike protein. This newfound rigidity helps “lock” the protein in one state or another, making it harder for the Spike protein to move between states.
These internal changes mean that oxidation doesn’t just increase the energy required for the Spike protein to change form; it also adds a layer of structural stability. This insight is essential because it shows that oxidation not only impacts the Spike protein’s flexibility but also makes the protein itself structurally more “stuck” in a specific position, hindering its ability to initiate infection.
How Could This Be Used in COVID-19 Treatment?
The implications of this research are far-reaching, especially in the field of COVID-19 treatments. Most current treatments focus on inhibiting viral replication or boosting the immune system’s response. However, this oxidation approach offers a fundamentally different strategy: it aims to prevent the virus from entering cells in the first place.
If researchers can develop safe methods to promote cysteine oxidation in the Spike protein, they might be able to reduce the virus's infectivity directly. This approach would work by limiting the virus’s ability to latch onto human cells, potentially stopping it from spreading or significantly lowering the severity of the infection. This method also has the potential to be less invasive than approaches that rely on altering the immune response, which can sometimes lead to unintended side effects. Instead of triggering immune activity, oxidation-based therapies would interfere directly with the viral structure, offering a more targeted, possibly safer approach to treatment.
Another exciting possibility is that this method could be adapted to work against other viruses with similar structural features, broadening its applicability in the fight against viral diseases.
Future Steps and the Path to Clinical Applications
Although this study was conducted through computer simulations, the findings offer a valuable basis for laboratory studies and, eventually, clinical trials. The next step would involve testing the oxidation process in a laboratory setting to determine if it can be safely and effectively applied to actual viral particles.
Researchers will also need to explore delivery methods that can safely introduce oxidizing agents to the Spike protein without affecting healthy cells.
If these oxidation effects are confirmed in a laboratory, they could revolutionize how we approach not only COVID-19 but potentially other viruses that rely on similar conformational flexibility to infect host cells.
The researchers’ findings highlight the importance of molecular simulation in developing new treatments, especially during pandemics when speed and innovation are crucial. By pinpointing the energy barriers and structural modifications that can limit viral infectivity, this study paves the way for new, targeted approaches to viral inhibition.
Conclusion
This research highlights an innovative path to combating SARS-CoV-2 by targeting the virus's structural vulnerabilities. The findings emphasize that oxidizing cysteine residues in the Spike protein stabilizes the protein in a less infective form, blocking the virus’s entry into human cells. This strategy provides an alternative approach to traditional antiviral therapies by focusing on structural, rather than immune-related, interventions.
By focusing on increasing the energy barriers to viral entry, this method could potentially reduce COVID-19’s infectivity and severity, leading to improved treatment outcomes. It also underscores the potential of chemical modifications to render viruses less able to infect cells, which could be a promising line of defense against future viral threats.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2024.10.24.620034v1
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/to-evade-the-immune-system-and-antibodies-sars-cov-2-rbd-switches-to-the-down-conformation
https://www.thailandmedical.news/news/breaking-covid-19-news-texas-study-finds-that-omicron-variants-utilize-conformational-changes-to-evade-neutralization-by-key-antibodies
