Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 05, 2024 5 months, 1 week, 1 day, 8 hours, 19 minutes ago
Medical News: A team of researchers from Istituti Clinici Scientifici Maugeri IRCCS in Pavia, Italy, the National Research Council (CNR)-IASI in Rome, and other renowned institutions has revealed that COVID-19’s effects extend far beyond the initial infection. Their findings indicate that the virus leaves a lasting legacy on our DNA, specifically through epigenetic modifications such as DNA methylation.
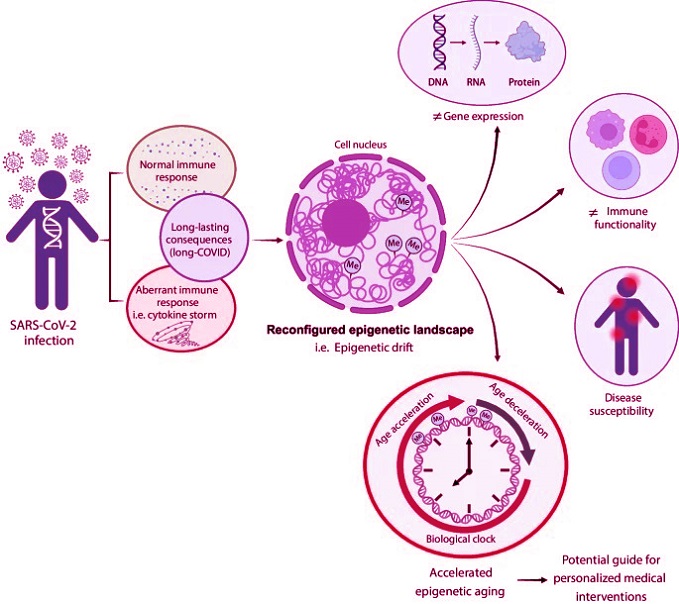 Graphical Abstract: Impact of SARS-CoV-2 infection on the epigenetic landscape and individual response
Following SARS-CoV-2 infection, individuals may develop either a normal immune response or an aberrant one, such as a cytokine storm. Both scenarios can result in long-lasting consequences, known as “long COVID.” This condition can reshape the epigenetic landscape by altering DNA methylation patterns, contributing to the “epigenetic drift.” This drift, further influenced by various factors, can lead to changes in gene expression, immune functionality, and disease susceptibility. One significant consequence of the epigenetic drift is the acceleration of biological aging, which can profoundly impact personalized medical interventions
Graphical Abstract: Impact of SARS-CoV-2 infection on the epigenetic landscape and individual response
Following SARS-CoV-2 infection, individuals may develop either a normal immune response or an aberrant one, such as a cytokine storm. Both scenarios can result in long-lasting consequences, known as “long COVID.” This condition can reshape the epigenetic landscape by altering DNA methylation patterns, contributing to the “epigenetic drift.” This drift, further influenced by various factors, can lead to changes in gene expression, immune functionality, and disease susceptibility. One significant consequence of the epigenetic drift is the acceleration of biological aging, which can profoundly impact personalized medical interventions
Epigenetics, the study of how behavior and environment influence gene activity, is central to this discussion. DNA methylation is a biochemical process that controls gene expression without altering the genetic code itself. This study explores how the virus's impacts on DNA methylation may influence health outcomes, aging, and potential therapies.
DNA methylation changes, when they occur in response to external stressors like viral infections, can have profound implications on health. Unlike genetic mutations, which alter the sequence of DNA, epigenetic changes influence how genes function without modifying the DNA itself. Researchers in this study emphasize that COVID-19 could drive long-term genetic shifts, contributing to an increased risk of health complications and accelerated aging. This
Medical News report delves into their discoveries and examines what it could mean for future health and therapeutic advances.
The Science of DNA Methylation: How COVID-19 Alters Our Epigenome
DNA methylation involves adding a small molecule, called a methyl group, to the DNA molecule, often at sites near genes that regulate immune function. This biochemical change either activates or silences genes, influencing various biological processes. In viral diseases like hepatitis and HIV, researchers have observed changes in DNA methylation that contribute to disease progression. In these instances, methylation patterns have been linked to increased risks of conditions such as cancer and autoimmune disorders. Now, scientists are investigating whether COVID-19 might have similar, potentially lasting effects on the epigenome.
Studies are showing that in COVID-19, DNA methylation changes target immune-related genes. These changes may alter immune responses and contribute to symptoms seen in severe cases of COVID-19, like cytokine storms. These storms - hyperactive immu
ne responses that can cause tissue and organ damage - are among the most severe consequences of COVID-19 infection. By understanding these epigenetic shifts, researchers are gaining insight into how COVID-19 may disrupt immune system functioning and predispose individuals to prolonged health issues, such as “long COVID,” and accelerated aging.
Epigenetic Drift: Accelerated Aging as a Result of COVID-19
One of the most surprising discoveries from the study was that COVID-19 could accelerate what is known as "epigenetic drift." This term describes the gradual changes in DNA methylation patterns that naturally occur as we age, contributing to biological aging and increasing vulnerability to age-related diseases. Researchers discovered that COVID-19 may speed up this process, leading to an epigenetic profile that resembles that of an older person.
For example, scientists found evidence of methylation changes in genes that play a role in inflammation and immune response, such as Interleukin 6 (IL-6) and Tumor Necrosis Factor-alpha (TNF-α). Altered methylation patterns in these genes could contribute to the immune system’s dysregulated response observed in severe COVID-19 cases. This acceleration of biological aging may leave survivors of severe COVID-19 more vulnerable to age-related diseases, a phenomenon that researchers describe as a “reconfigured epigenetic landscape.” In essence, the virus could leave a lasting imprint on survivors' DNA, contributing to premature aging and increased health risks over time.
Key Findings: Insights into COVID-19’s Long-Term Epigenetic Consequences
The research team focused on mapping the specific DNA methylation patterns in COVID-19 patients to understand how these changes might influence health. They observed that severe cases of COVID-19 often show significant alterations in methylation sites related to inflammation and immunity. In particular, genes associated with cytokine signaling - a key process in immune responses - appear to be heavily impacted by these epigenetic shifts. This could explain why some individuals experience intense immune reactions, while others have milder cases. Alterations in these genes may provide a biomarker for understanding and predicting which individuals are more likely to suffer severe outcomes.
The study also revealed changes in methylation around genes that code for ACE2 and TMPRSS2. ACE2 is the primary receptor that the SARS-CoV-2 virus uses to enter human cells, and TMPRSS2 is an enzyme that aids in the virus’s entry. Epigenetic changes to these genes could influence the body’s susceptibility to infection, potentially affecting how much virus enters the body or the severity of symptoms. This suggests that these methylation patterns might serve as a biomarker for both COVID-19 severity and susceptibility, providing clinicians with a new way to assess risk and personalize treatments.
Epigenetic Clocks: Measuring Accelerated Aging in COVID-19 Patients
Scientists used a specialized tool known as an "epigenetic clock" to measure the biological age of individuals based on their DNA methylation patterns. This method revealed that severe COVID-19 patients often exhibit an advanced biological age compared to their chronological age. By measuring this difference, researchers concluded that COVID-19 may speed up the aging process, particularly in the immune system, where changes in methylation reflect a heightened inflammatory response often associated with aging.
The epigenetic clock findings align with a broader trend: older adults are more vulnerable to severe COVID-19 symptoms. Researchers suggest this vulnerability could be due to their “older” epigenetic profiles, which predispose them to stronger inflammatory responses and immune system dysregulation. For those who survive severe COVID-19, these accelerated aging markers could indicate a higher risk for age-related diseases. Clinicians could potentially use epigenetic clocks as biomarkers to monitor long-term health in COVID-19 survivors, especially those experiencing persistent symptoms or complications.
The Role of Inflammation in COVID-19 and Epigenetic Changes
One of the core findings from the study is how COVID-19-related inflammation influences DNA methylation. Inflammation is the body’s natural response to infection; however, prolonged inflammation, especially as seen in severe COVID-19 cases, can have lasting effects on our DNA. Researchers noted that this inflammation could drive random (stochastic) changes in methylation, which can destabilize immune functions. These random changes accumulate over time, contributing to what scientists call “inflammaging” or age-associated inflammation. For older adults, whose bodies are already more prone to inflammaging, COVID-19 might worsen age-related health conditions.
Potential for Reversing COVID-19 Epigenetic Changes
Given these findings, scientists are now exploring whether it might be possible to reverse or mitigate the epigenetic impacts of COVID-19. Some therapeutic approaches, such as DNA methyltransferase inhibitors, are under investigation for their potential to counteract accelerated aging. These inhibitors, which are already used in cancer treatment, could theoretically help reduce the long-term effects of COVID-19 on DNA methylation. However, researchers caution that such treatments must be approached carefully due to the complexity of methylation patterns and the risk of unintended consequences. Specifically, broad changes in methylation could interfere with essential gene functions, potentially leading to other health issues.
The Future of COVID-19 Epigenetic Research and Its Implications for Health
This emerging research into COVID-19’s effects on DNA highlights a pressing need for further studies. By understanding how SARS-CoV-2 alters DNA, scientists could develop targeted therapies to counteract these changes. There is a hope that future studies will clarify the specific mechanisms by which COVID-19 induces DNA methylation changes, allowing for therapies that directly address these epigenetic shifts. This research could pave the way for preventive treatments, helping those at risk of severe COVID-19 to maintain a healthier epigenetic profile.
While epigenetic studies are still in their early stages, the potential impact on health and aging could be significant. For example, integrating DNA methylation biomarkers into regular health assessments might enable clinicians to personalize medical treatments. Patients with accelerated aging markers could benefit from therapies designed to slow down the epigenetic clock, possibly improving health outcomes and reducing susceptibility to age-related diseases.
Conclusion: COVID-19’s Legacy on Health and Aging
The research on COVID-19 and DNA methylation opens a new chapter in understanding the virus's lasting impacts on health. While the world has focused primarily on short-term symptoms and recovery, these findings reveal a deeper layer of long-term genetic consequences. COVID-19 appears to drive accelerated aging in those who survive severe cases, reshaping our understanding of its legacy. The study indicates that viral infections may leave a more enduring imprint on our health than previously realized. By mapping out these epigenetic changes, scientists could uncover new pathways to mitigate COVID-19’s lasting effects, paving the way for more effective treatments and preventive care.
The study findings were published in the peer-reviewed journal: GeroScience.
https://link.springer.com/article/10.1007/s11357-024-01406-7
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/study-finds-that-long-covid-linked-to-accelerated-aging-and-epigenetic-changes
https://www.thailandmedical.news/news/covid-19-accelerates-biological-aging
https://www.thailandmedical.news/news/breaking-covid-19-news-israeli-and-american-study-shows-epigenetic-changes-occurring-with-elevated-a-to-i-rna-editing-in-covid-19-infected-individuals
https://www.thailandmedical.news/news/philadelphia-study-validates-that-sars-cov-2-causes-mitochondrial-metabolic-and-epigenomic-reprogramming
https://www.thailandmedical.news/news/breaking-covid-19-news-polish-review-study-shows-that-sars-cov-2-infections-are-a-potential-risk-factor-for-the-development-of-cancer
https://www.thailandmedical.news/news/breaking-news-covid-19-exposure-during-pregnancy-leads-to-epigenetic-changes-in-newborns,-paving-the-way-for-health-issues-later-in-life
https://www.thailandmedical.news/news/study-finds-that-both-sars-cov-2-nucleocapsid-protein-and-nsp-13-protein-are-able-to-cause-human-host-dna-melting
https://www.thailandmedical.news/news/breaking-news-even-asymptomatic-sars-cov-2-infections-cause-dna-methylation-and-epigenetic-changes,-leading-to-immune-dysregulation
