COVID1-9 News - SARS-CoV-2 Infections Causes Gut Immunological Barrier Dysfunction Apr 08, 2023 2 years, 1 week, 3 days, 9 hours, 7 minutes ago
COVID-19 News: A new study by researchers from Medical School, University of Patras-Greece, University Hospital of Patras-Greece and Patras State General Hospital-Greece has found that SARS-CoV-2 infections often lead to the dysfunction of the gut’s immunological barrier.
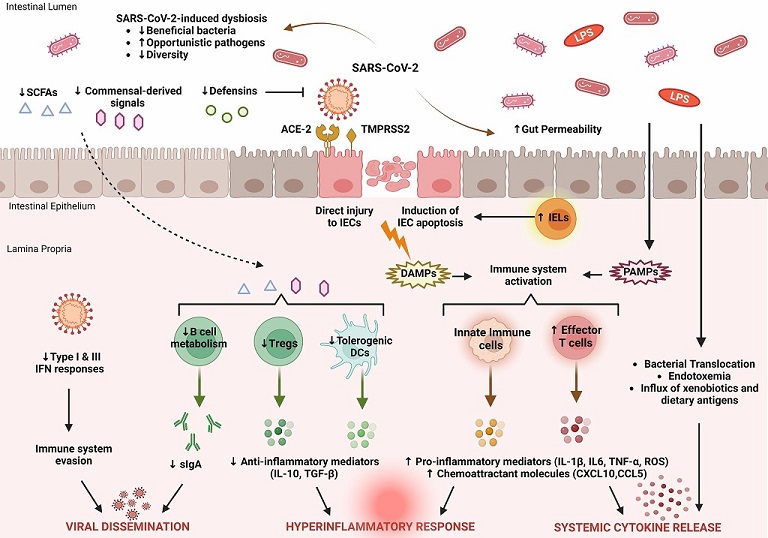 Key features of intestinal immune barrier disruption during SARS-CoV-2 infection. SARS-CoV-2 infection is associated with profound alterations in the intestinal microflora, manifested by decreased species diversity, depletion of symbiotic microorganisms, and prevalence of pathogenic species. Signals and metabolites derived from the intestinal flora, such as short-chain fatty acids (SCFAs), play an important role in controlling mucosal immunity by promoting T regulatory cell (Treg) responses and the activity of tolerogenic dendritic cells (DCs). This immunoregulatory environment, rich in anti-inflammatory mediators (IL-10, TGF-β), is significantly impaired by SARS-CoV-2. As a result, B cell metabolism and maturation are severely impaired, leading to exhaustion of effective plasma cells that produce secretory dimeric immunoglobulin A (sIgA), which is essential for viral containment. The proliferation of SARS-CoV-2 is also facilitated by its ability to evade recognition by the immune system by interfering with type I and type III IFN signaling. SARS-CoV-2 exerts either direct cytopathic effects on intestinal epithelial cells (IECs) expressing ACE2 and TMPRSS2 receptors or indirect immune-mediated injury. During COVID-19, the expression of several antimicrobial peptides, including defensins, is dysregulated, which increases the infectivity of SARS-CoV-2. In addition, recruitment of intraepithelial lymphocytes (IELs) accelerates IEC apoptosis. The release of damage-associated molecular patterns (DAMPs) due to cell injury and the influx of pathogen-associated molecular patterns (PAMPs) as a result of increased gut permeability lead to immune activation. Macrophages/monocytes, neutrophils, and other cells of the innate immune system secrete large amounts of proinflammatory mediators (IL-1β, IL-6, TNF-α, ROS) and chemokines (CCL5, CXCL10) that cause recruitment of additional immune cells and prime effector T cells. In parallel, disruption of the intestinal barrier facilitates bacterial translocation, endotoxemia, and dissemination of other gut-derived stimuli that contribute to systemic hyperinflammatory responses and cytokine release syndrome, leading to severe COVID-19.
Key features of intestinal immune barrier disruption during SARS-CoV-2 infection. SARS-CoV-2 infection is associated with profound alterations in the intestinal microflora, manifested by decreased species diversity, depletion of symbiotic microorganisms, and prevalence of pathogenic species. Signals and metabolites derived from the intestinal flora, such as short-chain fatty acids (SCFAs), play an important role in controlling mucosal immunity by promoting T regulatory cell (Treg) responses and the activity of tolerogenic dendritic cells (DCs). This immunoregulatory environment, rich in anti-inflammatory mediators (IL-10, TGF-β), is significantly impaired by SARS-CoV-2. As a result, B cell metabolism and maturation are severely impaired, leading to exhaustion of effective plasma cells that produce secretory dimeric immunoglobulin A (sIgA), which is essential for viral containment. The proliferation of SARS-CoV-2 is also facilitated by its ability to evade recognition by the immune system by interfering with type I and type III IFN signaling. SARS-CoV-2 exerts either direct cytopathic effects on intestinal epithelial cells (IECs) expressing ACE2 and TMPRSS2 receptors or indirect immune-mediated injury. During COVID-19, the expression of several antimicrobial peptides, including defensins, is dysregulated, which increases the infectivity of SARS-CoV-2. In addition, recruitment of intraepithelial lymphocytes (IELs) accelerates IEC apoptosis. The release of damage-associated molecular patterns (DAMPs) due to cell injury and the influx of pathogen-associated molecular patterns (PAMPs) as a result of increased gut permeability lead to immune activation. Macrophages/monocytes, neutrophils, and other cells of the innate immune system secrete large amounts of proinflammatory mediators (IL-1β, IL-6, TNF-α, ROS) and chemokines (CCL5, CXCL10) that cause recruitment of additional immune cells and prime effector T cells. In parallel, disruption of the intestinal barrier facilitates bacterial translocation, endotoxemia, and dissemination of other gut-derived stimuli that contribute to systemic hyperinflammatory responses and cytokine release syndrome, leading to severe COVID-19.
COVID-19 primarily presents with mild respiratory symptoms, but a subset of patients may develop severe complications, including multiple organ injury as reported in previous studies and
COVID-19 News coverages. The gastrointestinal (GI) tract can be directly infected by the SARS-CoV-2 virus or indirectly affected through viremia and the release of inflammatory mediators. Impaired intestinal barrier function is a key factor in SARS-CoV-2 infection, leading to excessive microbial and endotoxin translocation, strong systemic immune response, and the development of viral sepsis syndrome.
SARS-CoV-2 affects multiple components of the gut immune system, resulting in a diminished or dysfunctional gut immunological barrier.
Antiviral peptides, inflammatory medi
ators, immune cell chemotaxis, and secretory immunoglobulins are negatively affected. Mucosal CD4+ and CD8+ T cells, Th17 cells, neutrophils, dendritic cells, and macrophages are activated, and the number of regulatory T cells decreases, promoting an overactivated immune response with increased expression of type I and III interferons and other proinflammatory cytokines.
Furthermore, these changes may be promoted by a dysbiotic gut microbiota and a proinflammatory intestinal environment.
Gastrointestinal symptoms are reported in up to 24% of COVID-19 patients, including diarrhea, abdominal discomfort, nausea, vomiting, and loss of appetite.
Some patients may develop severe duodenitis and gastrointestinal bleeding. COVID-19 is associated with multifactorial impairment of the gut barrier, including deleterious effects on the gut microbiota, intestinal epithelial cells and their junctions, and gut-associated immune cells, immunoglobulins, and cytokine production.
The study found that intestinal barrier integrity is significantly impaired in COVID-19 patients, as evidenced by higher plasma levels of zonulin, indicating disruption of tight junction homeostasis, and increased levels of lipopolysaccharide-binding protein (LBP) and β-glucan, markers of bacterial and fungal translocation, respectively.
Serum markers of tight junction permeability and microbial translocation were significantly associated with circulating proinflammatory mediators, such as IL-6, suggesting that systemic inflammation is triggered by gut barrier disruption.
The study also showed that urinary intestinal fatty acid-binding protein (I-FABP) levels, a biomarker of intestinal injury, are elevated in patients with COVID-19.
Fecal calprotectin, a reliable marker of bowel inflammation, has been studied in COVID-19 patients. High fecal calprotectin levels are an independent risk factor for developing COVID-19 pneumonia, positively correlated with serum IL-6, hypoxemia, and hospitalization days. Serum calprotectin is an effective marker for predicting the future status of SARS-CoV-2-infected individuals.
Mass cytometric analysis of intestinal tissue from deceased COVID-19 patients revealed leukocytic infiltration, consisting of monocytes, CD11b+ macrophages, CD11c+ dendritic cells, natural killer cells, and B cells. Exhaustion/depletion of CD4+ T cells is also observed in SARS-CoV-2 infection, potentially contributing to intestinal epithelial barrier dysfunction and leaky gut, promoting systemic inflammation. IL-17 producing Th17 cells are overactivated in SARS-CoV-2 infection.
SARS-CoV-2 invasion of intestinal cells led to increased expression of type I and III interferons (IFN) and other proinflammatory cytokines. SARS-CoV-2 has developed several strategies to evade immune surveillance by attenuating type I and III IFN responses. Impairment of IFN-I-dependent immunity by any mechanism can lead to severe COVID-19 symptoms.
It has been found that upon intranasal SARS-CoV-2 infection, various cytokines and inflammatory mediators are initially produced in gastrointestinal tissues. Meanwhile, the digestive tract can upregulate anti-inflammatory IL-10 and inhibit pro-inflammatory IL-1β and IFN-γ. A gut-on-a-chip model and gene set enrichment analysis in pluripotent stem cells derived from small intestinal epithelial cells showed increased expression of cytokines and chemokines. Higher IL-8 and IL-18 and lower IL-10 levels were also found in the stool of COVID-19 hospitalized patients.
Short-chain fatty acids (SCFAs) can reduce pro-inflammatory mediators, but showed no effect on cell permeability. However, SCFAs modestly reduced the expression of interferon lambda receptor 1 (IFNLR1) and the serine protease TMPRSS2. Antibiotic-induced gut microbiota depletion did not impact mortality in a mouse model of SARS-CoV-2 infection, but increased colonic concentrations of IL-17 and CXCL2.
Soluble mucosal addressin cell adhesion molecule (sMAdCAM) is inversely correlated with serum IL-6 levels during SARS-CoV-2 infection. Lower sMAdCAM levels were observed in COVID-19 patients, suggesting its normalization may indicate restored mucosal homeostasis and emphasize its importance in therapeutic guidance and prophylactic intervention.
Regarding the gastrointestinal antiviral response, Paneth cells and neutrophils produce immunomodulatory defensins, which have diverse immunoregulatory functions and broad-spectrum antimicrobial and antiviral effects. In SARS-CoV-2 infection, α-defensin 5 shields the ACE2 receptor and prevents viral binding, offering protection due to its abundance in the digestive tract. β-defensin 1 production increases in later stages of infection due to intestinal hypoxia.
The gut's immunologic barrier is reinforced by secretory immunoglobulin A (sIgA), produced by mucosal lymphoid tissues. Commensal microorganisms play a crucial role in controlling IgA class switching and effective antibody production. The prevalence of sIgA in the intestine likely explains the attenuated gut inflammation compared to lung tissue. In vitro studies found that dimeric IgA was more effective in neutralizing SARS-CoV-2 than IgA monomers or IgG.
The gut microbiome plays a crucial role in maintaining immune system balance by interacting with immune cells, promoting tolerance to beneficial bacteria, and eliminating harmful species. COVID-19 has been linked to significant alterations in the gut microbiome, which may result from a severe systemic inflammatory response. The mechanisms behind these changes remain unclear, but they have been associated with interactions between the ACE2 receptor and SARS-CoV-2, leading to impaired secretion of antimicrobial peptides (AMPs) and disruptions in the gut microbiota composition.
Dysbiosis during SARS-CoV-2 infection is characterized by a reduced ratio of Firmicutes to Bacteroidetes, a decrease in butyrate-producing bacterial species like Faecalibacterium and Roseburia, and a reduction in other beneficial bacterial genera. These changes in the gut microbiome have been associated with COVID-19 severity, suggesting a potential prognostic role for these alterations. A dynamic process underlies the changes in the gut microbiota of COVID-19 patients, and the gut microbiota's regulatory functions may support recovery from SARS-CoV-2 infection.
COVID-19 impacts multiple systems, including the gastrointestinal tract and gut barrier integrity. The severity of the disease is reflected in the extent of disruptions to the mucosal immune barrier. SARS-CoV-2 evades the innate immune response by disrupting interferon signaling and severely affecting cytokine expression in the mucosal compartment. Additionally, the production and release of antimicrobial peptides and secretory IgA, important regulators of intestinal immune integrity, are impaired. This results in significant alterations to the gut microbiome and metabolome, marked by the depletion of symbiotic species and the dominance of pathogenic microorganisms.
Prognostic factors related to the gut immunological barrier have been evaluated and found to have strong associations with disease severity, poor prognosis, hospitalization, or mortality due to SARS-CoV-2. A prognostic scoring system incorporating these factors with other epidemiologic, clinical, and laboratory data could provide the most accurate predictions. Further studies are needed to identify markers of gut barrier dysfunction for high-risk COVID-19 patients requiring early or enhanced support.
In conclusion, SARS-CoV-2 infection disrupts intestinal immunological homeostasis, impairs mucosal immune cell function, and alters signaling molecule production…all affecting the gut immunological barrier significantly. These changes could be driven by SARS-CoV-2-induced gut dysbiosis and the ongoing dialogue between the mucosal immune system and gut microflora. Dysregulation of intestinal immune cells and overproduction of proinflammatory cytokines can compromise intestinal epithelial cell integrity and connections, leading to gut barrier dysfunction. This may result in microbial and endotoxin translocation into the systemic circulation, promoting hyperinflammatory responses, distant organ dysfunction, and a "viral sepsis syndrome." The prognostic potential of gut immunologic barrier alterations in COVID-19 highlights their importance. More clinical studies are needed to explore the role of biomarker-based immunologic therapies in improving gut barrier function and expanding COVID-19 treatment options.
The study findings were published in the peer reviewed journal: Frontiers In Immunology.
https://www.frontiersin.org/articles/10.3389/fimmu.2023.1129190/full
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
