COVID-19 Causes Sugar Modifications of IgA, Which in Turn Contributes to NETosis
Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 25, 2024 5 months, 2 weeks, 5 days, 3 hours, 40 minutes ago
Medical News: Researchers have uncovered critical differences in how the immune system behaves in severe cases of COVID-19, revealing a potential link to blood clot complications seen in critically ill patients. The study focuses on a key immune component - immunoglobulin A (IgA) - and its changes during severe infections, especially in cases of COVID-19 and influenza.
 COVID-19 Causes Sugar Modifications of IgA, Which in Turn Contributes to NETosis
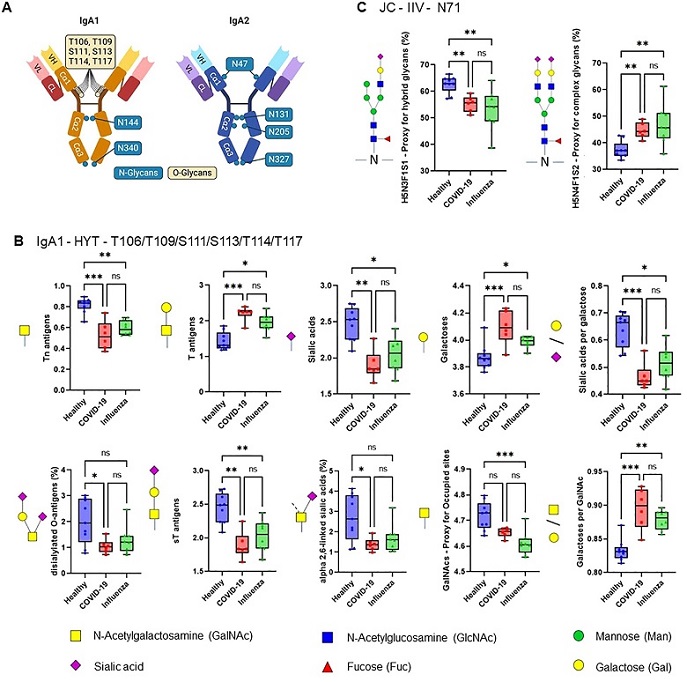
ARDS IgA glycosylation patterns differ from those of healthy controls. (A) Schematic representation of plasma IgA1/2. IgA has two heavy (H) chains and two light (L) chains connected by disulphide bridges. The heavy chains are composed of three constant (Cα) domains and one variable (V) domain, and the light chains are composed of one C domain and one V domain. Locations of known IgA N- and O-glycans are shown. Created with biorender.com. (B, C) Differences in plasma IgA glycosylation profiles between healthy and patients with COVID-19 or Influenza ARDS for indicated clusters and glycosylation sites (B, IgA1 - HYT - T106/T109/S111/S113/T114/T117; C, JC - IIV - N71). Differences in plasma IgA glycosylation patterns were assessed using the Kruskal-Wallis test followed by uncorrected Dunn’s test. ***p<0.001; **p<0.01; *p<0.05; ns, not significant.
COVID-19 Causes Sugar Modifications of IgA, Which in Turn Contributes to NETosis
ARDS IgA glycosylation patterns differ from those of healthy controls. (A) Schematic representation of plasma IgA1/2. IgA has two heavy (H) chains and two light (L) chains connected by disulphide bridges. The heavy chains are composed of three constant (Cα) domains and one variable (V) domain, and the light chains are composed of one C domain and one V domain. Locations of known IgA N- and O-glycans are shown. Created with biorender.com. (B, C) Differences in plasma IgA glycosylation profiles between healthy and patients with COVID-19 or Influenza ARDS for indicated clusters and glycosylation sites (B, IgA1 - HYT - T106/T109/S111/S113/T114/T117; C, JC - IIV - N71). Differences in plasma IgA glycosylation patterns were assessed using the Kruskal-Wallis test followed by uncorrected Dunn’s test. ***p<0.001; **p<0.01; *p<0.05; ns, not significant.
The research team, which included scientists from leading institutions such as the Universities of Giessen and Marburg Lung Center in Germany, the Leiden University Medical Center in the Netherlands, and Hannover Medical School in Germany, found unique patterns in the way IgA behaves during these infections. Their findings might explain why some COVID-19 patients experience dangerous blood clots, a condition known as immunothrombosis.
What Is IgA, and Why Does It Matter?
IgA is an antibody that plays an important role in the body’s immune response, especially in protecting against infections that attack the respiratory and digestive systems. However, when IgA’s function is disrupted, it can have dangerous effects on the body’s ability to defend itself, potentially leading to complications like blood clots.
This
Medical News report details new research that focused on changes in the glycosylation of IgA in patients suffering from severe COVID-19 and acute respiratory distress syndrome (ARDS) caused by influenza. Glycosylation is a process that modifies proteins like IgA by attaching sugar molecules, which in turn affects their function in the immune system. The study discovered that these modifications, or glycosylation patterns, were different in COVID-19 patients compared to those with severe influenza.
Study Details
The study involved 28 participants, including 10 critically ill COVID-19 patients, eight patients with ARDS caused by influenza, and 10 healthy volunteers. All patient samples were taken within two days after the onset of ARDS symptoms. Plasma IgA was isolated and analyzed for its glycosylation pattern
s, while NET formation was assessed using laboratory assays.
Key Differences in Immune Response
The research team analyzed blood samples from three groups of people: critically ill COVID-19 patients, those with ARDS caused by influenza, and healthy volunteers. One of the key findings was that COVID-19 patients showed a lower level of sialylation, a type of sugar modification on IgA, compared to healthy individuals. Instead, they had a higher level of galactosylation, another sugar type. These changes in glycosylation seem to be linked to how IgA interacts with the immune system during severe illness.
The researchers also discovered that these altered IgA molecules in COVID-19 patients have a stronger ability to induce a process called NETosis, which involves immune cells called neutrophils releasing web-like structures, known as neutrophil extracellular traps (NETs). While NETs are usually helpful in trapping and neutralizing harmful microbes, too many of them can lead to excessive blood clotting. This is particularly significant in COVID-19, where blood clots have been a major complication.
How COVID-19 Differs from Influenza
When comparing the blood samples of COVID-19 patients with those suffering from severe influenza, the researchers found that the glycosylation patterns were distinct. For example, a specific sugar modification at site N47 of IgA2 was more prevalent in influenza patients, while COVID-19 patients had lower levels of other complex sugars in their IgA1 and IgA2 proteins.
This is important because it suggests that COVID-19 triggers a unique immune response that might explain why patients with the disease are at a higher risk of developing blood clots. The researchers also found a strong correlation between these glycosylation changes and the levels of extracellular DNA (exDNA) in the blood of COVID-19 patients, a marker that indicates the presence of NETs.
What Does This Mean for Patients?
The discovery that IgA behaves differently in COVID-19 compared to other viral infections like influenza is a significant step forward in understanding the disease. Blood clots and thromboembolic complications have been a common and dangerous issue in severe COVID-19 cases. The new findings suggest that the glycosylation changes in IgA could be a contributing factor to this increased risk.
Further research will be needed to fully understand how these changes in IgA affect the body and whether they could be used to develop new treatments. If scientists can find a way to prevent these harmful glycosylation changes, it may be possible to reduce the risk of blood clots in critically ill COVID-19 patients.
Conclusions and Future Directions
In conclusion, the study sheds new light on how COVID-19 affects the immune system, particularly through changes in IgA glycosylation. These modifications appear to make COVID-19 patients more prone to developing harmful blood clots by increasing the tendency of immune cells to undergo NETosis.
The research emphasizes the need for further investigation into how these glycosylation patterns impact other aspects of the immune response and how they might be targeted for therapeutic interventions. Understanding these processes could lead to better treatments for severe COVID-19 and other infectious diseases where immune responses go awry.
The study’s findings suggest that therapies designed to modify IgA glycosylation could be a promising avenue for preventing thromboembolic complications in COVID-19. In particular, future research could explore the possibility of using drugs that target specific glycosylation pathways to reduce the risk of blood clots in critically ill patients.
Given the role of NETosis and immunothrombosis in COVID-19, treatments that can reduce the production of NETs or counteract their harmful effects may also help improve outcomes for patients. Ultimately, this research brings us closer to understanding the complex immune processes that contribute to the severe complications seen in COVID-19, with the hope of finding more effective ways to treat and prevent these outcomes.
The study findings were published in the peer-reviewed journal: Frontiers in Immunology.
https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1439248/full
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/breaking-news-gene-study-reveals-how-covid-19-mrna-vaccine-leads-to-high-risk-of-iga-nephropathy-eventual-kidney-failure-and-possible-cancers
https://www.thailandmedical.news/news/scientists-from-portugal-find-that-diminished-peripheral-cd8-7-integrin-t-cells-and-anti-sars-cov-2-iga-response-characterizes-long-covid
