COVID-19 Clinical Trials: University of California Initiates Trial To Test Safety And Efficacy Of Convalescent Plasma For COVID-19 Prevention
Source: COVID-19 Clinical Trials Jul 10, 2020 4 years, 8 months, 3 weeks, 2 days, 20 hours, 18 minutes ago
COVID-19 Clinical Trials: Medical researchers from the University of California San Diego School of Medicine have launched a clinical trial to assess the safety and efficacy of convalescent plasma to prevent COVID-19 after a known exposure to the virus.
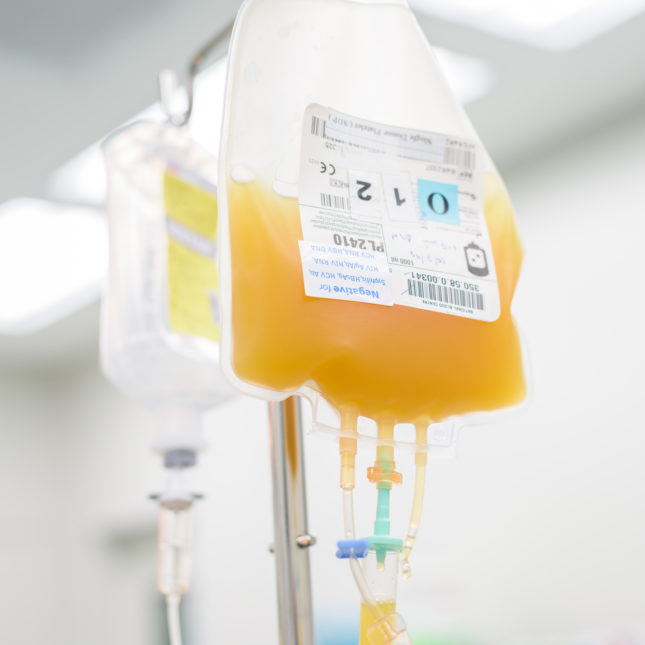
Convalescent plasma therapy involves infusing patients with antibodies extracted from the blood of donors who have successfully recovered from COVID-19, with the hope that the resulting boost to their immune systems will shorten the length and reduce the severity of the disease.
The University of California San Diego trial is part of a larger, national effort approved by the U.S. Food and Drug Administration. The goal is to create a network of hospitals and blood banks collecting, isolating, processing and testing whether plasma from COVID-19 survivors has therapeutic, preventive value. The national trial is being coordinated by Johns Hopkins University and sponsored by the National Institute of Health through the Department of Defense.
Dr Edward Cachay, MD., Professor, Department of Medicine, UC San Diego School of Medicine told Thailand Medical News, With convalescent plasma therapy, we want to act prophylactically, using a product with known high-titers or concentrations of neutralizing antibodies. We want to learn how we can prevent sickness, how we can prevent COVID patients from needed mechanical ventilation, and how we can prevent them from dying from the disease."
Dr Cachay is also an infectious disease specialist at UC San Diego Health.
Before the emergence of antibiotics, convalescent plasma was used to prevent and treat a host of bacterial and viral infections, including diphtheria, scarlet fever and pertussis.
Convalescent plasma was used during the 1918 influenza pandemic with reported good effect. In general, convalescent plasma treatment has proven safe, but its effectiveness has varied with disease and among individuals. Studies of convalescent plasma therapies for Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS) and the 2009 H1N1 influenza showed measurable reductions of mortality (compared to placebo or no therapy), but efforts to treat Ebola virus infections during the 2014-16 outbreak in West Africa were inconclusive.
Researchers from China treating COVID-19 patients have reported some success using convalescent plasma, albeit not in randomized, controlled studies ie the gold standard in clinical research.
The US FDA issued research guidelines in April for assessing convalescent plasma as a potential COVID-19 treatment and the American Red Cross is currently seeking blood plasma donors who have fully recovered from novel coronavirus infections.
Blood plasma is the liquid portion of blood that carries blood components throughout the body, such as red and white blood cells, platelets, salts and enzymes.
Plasma also contains proteins and antibodies produced by the body's immune system to fend off invasive pathogens, such as SARS-CoV-2.
In order to qualify as a plasma donor for COVID-19 patients, donors must be at least 17 years old and weigh 110 pounds; be in good health; and have a prior, verified diagnosis of COVID-19 but are now symptom-free and fully recovered.&l
t;br />
The University of California San Diego Health clinical trial will recruit a total of 487 qualifying participants for the study.
Key criteria to qualify for participation include a high-risk factor, such as age or an underlying condition, like cardiovascular disease, diabetes, existing pulmonary impairment or employment as a health care worker; known exposure to SARS-CoV-2; and a negative PCR diagnostic test to show no current infection.
Clinical testing will be conducted inside tents set up across from the emergency department at Jacobs Medical Center and the Altman Clinical and Translational Research Institute (ACTRI) on the La Jolla health campus.
The University of California Health Blood Bank is coordinating efforts with the San Diego Blood Bank.
The university is providing personnel, infrastructure support and other resources for the convalescent plasma trial and for other COVID-19-related clinical trials at University of California San Diego.
ACTRI has also created a COVID-19 Biobank to provide materials for research projects to diagnose or treat the disease.
Typically in cases of infection by the novel coronavirus, it appears the human immune system begins producing antibodies to the disease five to 10 days after the initial infection.
These antibodies bind to the targeted coronavirus, stopping it from latching onto new cells and beginning the production of more viral particles.
It has been observed that over the course of two or so weeks, the body clears out the virus, but antibodies to it (or the blueprints for making them) remain. However the depth and length of subsequent immunity have not been determined.
Dr Cachay said he thinks convalescent plasma will likely be most effective in persons with early exposure to the novel coronavirus, before symptoms appear, but it will require a clinical trial to substantiate that thinking.
He added, "If we do not do this, if we just gather anecdotal evidence that is not conclusive, then we will not be any better off when the next wave hits."
For more on
COVID-19 Clinical Trials, keep on logging to Thailand Medical News.
